
Where do new drugs come from? Why does it take so long to get a new drug approved? Why are drugs so expensive?
These are all good questions, and the answers can be found on this page, which talks about drug discovery and development.
- A Brief History of Drug Discovery and Development
- Sources of Drugs
- Pros and Cons of Natural and Synthetic Drugs
- Examples of Cancer Drugs
- Screening for Drug Leads
- Safety Testing of Drug Candidates
- Optimization and Pre-Clinical Tests
- Investigational New Drug (IND) Applications
- Clinical Trials
- New Drug Application (NDA)
- Post Approval: Manufacturing and More Research
- Financial Aspects of Drug Development and Sale
A Brief History of Drug Discovery and Development
The earliest writings on medicinal drug usage dates back to approximately 2500 BC.The following is a brief timeline of medicinal drug milestones.
- Circa 2500 BC
- Pen T'Sao - Chinese book by Emperor Shen Nung on medicinal roots and grasses 1

Pen T'Sao
- 1550BC
- Ebers Papyrus - Collection of 800 prescriptions for medicinal plant usages of garlic, willow, juniper, aloe, and more 1

Ebers Papyrus
- Prior to 20th century
- Medicine was made from crude and semi-pure plant, animal, microbe, and mineral extracts 2
- 20th century
- The receptor theory of drug action concluded that chemicals in extracts are responsible for a drug's biological activity. This theory helped elucidate the chemical structures of active ingredients in natural extracts, which led to the rise of synthetic chemistry 3
- Scientists began using more non-natural sources (synthetic and semi-synthetic) in drug discovery and development 4
- Advent of new, revolutionary technologies for discovering and developing potential therapeutic compounds, such as automated separation techniques, high-throughput screening, and combinatorial chemistry 5, 6
Sources of Drugs
The medicines we ingest, inject, and inhale are often complex therapeutic compounds. The drugs are usually mixtures of chemicals made from starting materials or drug sources. Depending on the sources from which the drugs were created, the drugs can be categorized as natural, synthetic, or semi-synthetic. 7
Natural drugs are made from compounds found in nature. The most prevalent natural drug sources are plants. The field of science that studies the relationship between people and medicinal plants is known as medicinal ethnobotany. Some examples of medicine that come from plants are morphine (from opium), digoxin (from flower, Digitalis lanata), and aspirin (from willow tree bark). Less prevalent natural drug sources include animals, microbes, and minerals. 7

Opium Poppy

Digitalis lanata

Willow Tree

From left to right: a) Structure of digoxin b) Structure of morphine c) Structure of acetylsalicylic acid (aspirin)
Synthetic drugs come from starting materials that are not found in nature. Instead, they are produced by man from smaller chemical building blocks. 7 An example of synthetic medicine is the experimental anti-malaria drug, arterolane. 8
Semi-synthetic drugs are neither completely natural nor completely synthetic. They are a hybrid. Semi-synthetic drugs are generally made by converting starting materials from natural sources into final products via chemical reactions. Examples of semi-synthetic medicine include the antibiotic, penicillin, and the chemotherapy drug, paclitaxel. To make the chemotherapy drug, paclitaxel, 10-deacetylbaccatin is extracted from yew needles and undergoes a 4-stage synthesis process. 7

Yew needles

Structure of 10-deacetylbaccatin

Structure of paclitaxel
In general, the discovery of natural drugs involves screening for and identifying active compounds in extracts created from plants, animals, microbes, and minerals. The development of synthetic drugs involves producing pure, isolated chemical compounds with the help of computer-based research and large collections of chemicals. The goal of both drug discovery processes is to identify 'leads'- chemicals that may have therapeutic effects and treat medical conditions including cancer, infections, high blood pressure, nervous system diseases and metabolic diseases. 7
Pros and Cons of Natural and Synthetic Drugs
Advantages of using natural drug sources 7
- More structural diversity and novelty compared to synthetic compounds
- Many natural chemicals are able to interact with proteins, and other biological molecules
- More complex in structure than synthetic molecules. This complexity allows for more selective binding to targets.
Disadvantages of using natural drug sources 7
- More time consuming
- More costly
- May be less sustainable
- Natural chemical compounds may work differently than expected once isolated from their source
Advantages of using synthetic drug sources 7
- Less time consuming
- Less costly
- May be more sustainable
Disadvantages of using synthetic drug sources 7
- They tend to have fewer therapeutic effects
- Many synthetic drugs cause unacceptable side effects.
Learn about the side effects of cancer treatment
Examples of Cancer Drugs
Methotrexate (MTX)
- Category
- Synthetic
- Mechanism of action
- Anti-folate - Blocks cancer cell growth by preventing cancer cells from using folate to make nucleic acids (DNA and RNA) 9
- Competes with folate for binding to the dihydrofolate reductase enzyme, preventing dihydrofolate from being converted into tetrahydrofolate (an essential ingredient in DNA synthesis and repair) 10
- Development
Learn more about methotrexate.
Learn about cancer drug resistance against methotrexate.
Sorafenib (Nexavar)
- Category
- Synthetic 12
- Mechanism of action
- Development
- Several synthetic mechanisms, but the most accepted method involves reaction of isocyanate with aniline using dichloromethane as the reagent. This produces a 92% yield. 12
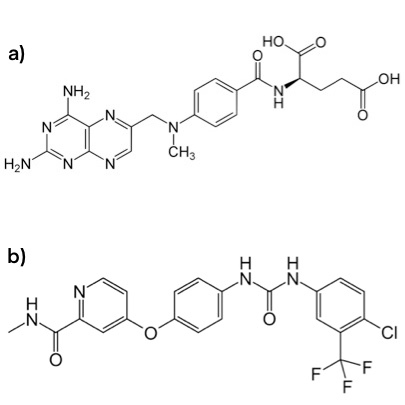
Structure of a) methotrexate b) sorafenib (Nexavar)
Vinblastine
- Category
- Mechanism of action
- Stops tumor growth by disrupting microtubule formation and inhibiting cell division. Microtubles are part of the cytoskeleton and are important in cell division. Vinblastine binds to small protein building blocks that are used to form microtubules and prevents them from forming long chains. 14
- Development

Madagascar periwinkle

Structure of vinblastine
Screening for Drug Leads
Natural drugs
During the early stages of the drug discovery and development process, scientists must select the most promising chemicals (called 'leads') to further study. They do this by isolating and purifying the lead compounds from their natural sources. The methods and equipment involved in this step depend on the structure, stability, and amount of the compound needed. In the case of penicillin, freeze-drying and chromatography are two of the techniques used. 7
To find the most promising lead compounds, scientists must screen for compounds that will be further studied. In order to efficiently screen a large number of compounds, high throughput screening (HTS) is typically used. In HTS, machines are used to test thousands of chemicals against specific targets. HTS has become a standard method for conducting millions of tests that help to identify the effects of possible drugs on biological pathways. 7
After the necessary biochemical and pharmacological tests have been conducted, scientists narrow down the list of compounds to include only the lead compounds that have the ability to react with target molecules in the desired way. At this stage in the drug discovery process, most lead compounds are not selective enough for their target molecule. In order to increase their selectivity, medicinal chemists alter the compounds' structures according to predicted structure-activity relationships. If the alterations improve selectivity, the lead compounds may be able to move onto in vitro and in vivo testing in selected disease models. 7
Synthetic drugs
The general stages of synthetic drug discovery are similar to those of natural drug discovery. The main difference between the two is the origin of the lead compounds. In natural drug discovery, the lead compounds come from natural sources (i.e. plants), while in synthetic drug discovery, the lead compounds are typically generated in labs by combinatorial chemistry, a technique by which hundreds to millions of molecules are created from smaller chemical building blocks.
Combinatorial chemistry is used in labs to create a large number of desired molecules. This method is an efficient way to make many new chemicals, but generates molecules that are less structurally complex than natural compounds. A more recently developed synthesis technique is called DNA-templated synthesis. This novel method allows researchers to more efficiently screen for compounds with desirable interactions with other biomolecules and also produce compounds that may not be produced via standard techniques. At this time, synthetic drug discovery has lower hit-rates via HTS because the products are not as diverse as those in natural products. 7
Safety Testing of Drug Candidates
After successfully selecting and screening for lead compounds, scientists must conduct safety tests on them to determine whether or not to proceed in the drug discovery and development process. Specifically, it is important to know how the drug is absorbed, distributed, metabolized, and excreted (ADME). The ADME test provides insight on what happens when to the drug in the body, a field of study called pharmacokinetics. 17Promising lead compounds must be non-toxic and able to be absorbed into the bloodstream, distributed to target sites in the body, metabolized effectively, and excreted from the body. 18

ADME diagram from Perkin Elmer
Optimization and Pre-Clinical Tests
Lead Compound Optimization
The purpose of lead compound optimization is to lessen any negative side effects associated with lead compounds and to make them more effective and selective. Scientists optimize lead compounds by changing their structures and testing these variations (called analogues). If optimization is successful, lead compounds can continue on in the drug discovery and development process, where they become potential candidate drugs. 18
Safety Screening
This stage in the drug discovery and development process has several goals. Scientists must determine if the candidate drugs are safe for human testing, understand exactly how the drug works, and determine effective and efficient ways of producing large quantities of the candidate drugs - a necessary process if the candidate drugs continue onto the clinical trial phases. Candidate drugs that pass safety tests are eligible for clinical trial applications. 18
Investigational New Drug (IND) Applications

The purpose of this stage in the drug discovery and development process is to determine whether or not candidate drugs can be used in clinical trials. The FDA and Institutional Review Board (IRB) must approve IND applications before clinical trials can begin. The FDA reviews all prior work to ensure that clinical trials will be reasonably safe and that there are no unreasonable risks. The IRB reviews the clinical trial plans that detail how trails will be conducted, which institutions, researchers, and doctors will be involved, and the informed consent procedure. If the IND application is approved, the candidate drug moves onto clinical trials. 18
Clinical Trials
After laboratory and animal tests are completed successfully, potential drugs are then run through a series of trials in human volunteers. Drugs that work well in other animals may not work in humans or they may have side effects that may show up in humans but not in the test animals. These trials also compare existing treatments to the new, test drug. Clinical trials are composed of several steps, or phases, described below.
Phase 0
This phase is designed to eliminate certain candidate drugs that are deemed ineffective. This is done via "microdosing" - small doses of a candidate drug are given to a few human volunteers, and the drug's effects are analyzed. The candidate drugs that pass this phase move onto phase I. Not all drugs go through phase 0 testing. 18
Phase I
This phase is designed to determine if a candidate drug is safe in humans. A small group of human volunteers (20 to 100 people) is given the candidate drug and the drug's effects are analyzed. Scientists will study the pharmacokinetics (ADME, side effects, desired effects, safe dosages, etc.) during this phase. The candidate drugs that pass this phase can move onto phase II. 18
Phase II
This phase is designed to determine the effectiveness of a candidate drug in a larger group of patients (100 to 500 people) who have the disease under study. The candidate drug's short-term side effects, risks, mechanism of action, drug efficacy, optimal dosages and treatment schedule are analyzed. The candidate drugs that pass this phase move onto phase III. 18
Phase III
This phase is designed to determine the safety, efficacy, and advantages versus disadvantages of the candidate drug in a larger group of patients (1,000 to 5,000 people). The information collected during this phase will help create the instructions for the drug's usage directions (risks, potential interactions with other drugs, etc.). The candidate drugs that pass this phase are eligible for the New Drug Application (NDA). 18
New Drug Application (NDA)
After phase III clinical trials, the candidate drug's sponsoring company analyzes all of the data collected from clinical trials to determine if the candidate drug is safe and effective enough to continue onto the next step: an NDA for approval by the FDA to market the candidate drug. The NDA is a collection of information that includes candidate drug studies, manufacturing proposals, and possible medicine labeling. The FDA, along with FDA-appointed experts, reviews the information to determine if the drug can be approved as a new medicine. There are three possible outcomes:
- The FDA can approve the candidate drug as a new medicine.
- The FDA can request more information and/or studies from the drug company before making a final decision.
- The candidate drug can be denied approval.
Post Approval: Manufacturing, Phase IV and Continued Research
If the FDA approves the candidate drug as a new medicine, the drug company can then begin the complicated and costly process of manufacturing the drug on a large scale. They must also abide by the FDA guidelines for Good Manufacturing Practices (GMP). 18 Phase IV, the long-term surveillance of the drug's effects on patients, begins as soon as the drug has been administered to patients in a non-trial setting. Periodically, the drug's sponsoring company must submit reports to the FDA detailing the efficacy and safety of the drug as well as any reported concerns surrounding the drug. 19
Financial Aspects of Drug Development and Sale
Many possible drugs that are identified early in the drug discovery and development process do not make it to the end. The entire process for discovering and developing a successful drug is extremely expensive and can last for more than a decade. According to a 2014 study conducted by the Tufts Center for the Study of Drug Development, successfully developing a new prescription medicine costs approximately $2.558 billion. An estimated $1.395 billion of this covers out-of-pocket expenses, while the remaining $1.163 billion covers the time cost (money investors forego while a drug is being developed). This 2014 total cost represents a 145% increase from the reported cost in 2003. The following are some possible factors that may play a role in the observed increase in drug development costs over the years
- larger and more complex clinical trials
- higher failure rates for drugs tested in humans
- protocol changes to include health technology assessment data. 20, 21
When a drug looks promising, the company will typically file for a patent to protect their product from competition. In the U.S., drug patents last 20 years. 22 Since companies often file for patents several years before their drugs are approved, they may end up only having a decade's worth of time to split even and earn profits, before the patents expire. The extremely high cost associated with bringing a drug from the laboratory to people is one reason why drugs are often very expensive. 21
- 1ab Petrovska BB. Historical review of medicinal plants' usage. Pharmacogn Rev. 2012 Jan;6(11):1-5. [PUBMED]
- 2 Historical Background to Drug Discovery. UGA Center for Drug Discovery. [http://cdd.rx.uga.edu/index.php/history/]
- 3 Maehle AH, Prull CR, Halliwell RF. The emergence of the drug receptor theory. Nat Rev Drug Discov. 2002 Aug;1(8):637-41. [PUBMED]
- 4 Historical Background to Drug Discovery. UGA Center for Drug Discovery. [http://www.uga-cdd.org/background.php]
- 5 Pereira DA, Williams JA. Origin and evolution of high throughput screening. Br J Pharmacol. 2007 Sep;152(1):53-61. Epub 2007 Jul 2. [PUBMED]
- 6 Pandeya SN, Thakkar D. Combinatorial chemistry: A novel method in drug discovery and its application. Indian Journal of Chemistry. Vol. 44B, Feb 2005, pp. 335-348. [http://nopr.niscair.res.in/bitstream/123456789/8939/1/IJCB%2044B(2)%20335-348.pdf]
- 7abcdefghijklm Lahlou M. The Success of Natural Products in Drug Discovery. Pharmacology & Pharmacy, 2014, 4, 17-31.
- 8 Valecha N, Looareesuwan S, Martensson A, Abdulla SM, Krudsood S, Tangpukdee N, Mohanty S, Mishra SK, Tyagi PK, Sharma SK, Moehrle J, Gautam A, Roy A, Paliwal JK, Kothari M, Saha N, Dash AP, Bjorkman A. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: a phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis. 2010 Sep 15;51(6):684-91. [PUBMED]
- 9 Methotrexate. American Cancer Society. [http://www.cancer.org/treatment/treatmentsandsideeffects/guidetocancerdrugs/methotrexate]
- 10ab Skubisz MM, Tong S. Of leaves and butterflies: how methotrexate came to be the savior of women. Obstet Gynecol. 2011 Nov;118(5):1169-73. [PUBMED]
- 11 WHO Model List of Essential Medicines. [http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf?ua=1]
- 12ab Zhang L, Xia W, Wang B, Luo Y, Lu W. Convenient Synthesis of Sorafenib and its Derivatives. Synthetic Communications, Vol 41 Issue 21, 2011.
- 13ab Sorafenib tosylate. National Cancer Institute. [http://www.cancer.gov/drugdictionary?cdrid=299013]
- 14abcd Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues. J Am Chem Soc. 2009 Apr 8;131(13):4904-16. [PUBMED]
- 15ab Vinblastine. CancerQuest. [http://www.cancerquest.org/patients/drug-reference/vinblastine]
- 16 Dr. Robert Laing Noble. The Canadian Medical Hall of Fame. [http://www.cdnmedhall.org/laureate-list]
- 17 Eddershaw PJ, Beresford AP, Bayliss MK. ADME/PK as part of a rational approach to drug discovery. Drug Discov Today. 2000 Sep;5(9):409-414. [PUBMED]
- 18abcdefghi Drug Discovery and Development: Understanding the R&D Process. Innovation.org. [http://www.innovation.org/drug_discovery/objects/pdf/RD_Brochure.pdf]
- 19 Suvarna V. Phase IV of Drug Development. Perspect Clin Res. 2010 Apr;1(2):57-60. [PUBMED]
- 20 DiMasi JA, Grabowski HG, Hansen RA. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of Health Economics 2016;47:20-33. [PubMed]
- 21ab Herper, Matthew. How Much Does Pharmaceutical Innovation Cost? A Look At 100 Companies. Forbes. [http://www.forbes.com/sites/matthewherper/2013/08/11/the-cost-of-inventing-a-new-drug-98-companies-ranked/]
- 22 Frequently Asked Questions on Patents and Exclusivity. U.S. Food and Drug Administration. [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/ucm079031.htm]
