Sorafenib
Approved in late 2005 for use in advanced renal cell (kidney) cancer. Approved in November 2007 for treatment of inoperable liver cancer. Sorafenib is also used in treatment of unresectable hepatocellular cancer (HCC). Sorafenib is administered as an oral capsule.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Sorafenib (Nexavar®) is characterized as a signal transduction inhibitor. This drug blocks multiple kinases involved in cell division and inhibits many growth factor receptors such as receptor tyrosine kinases.These receptor tyrosine kinases include PDGFR-beta, VEGFR-2, VEGFR-3, KIT, and FLT-3, which act to receive signals that directly or indirectly stimulate tumor growth. Blocking of the activity of these receptors can cause cancer cell death and/or a reduction in angiogenesis, the process that brings new blood vessels to a growing tumor. Sorafenib also inhibits other signal transduction proteins, enzymes (serine/threonine kinases) that help to stimulate cell division. Targets include CRAF, BRAF and mutated forms of BRAF.1
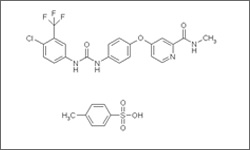
The diagram below shows the 3D molecular structure for Sorafenib.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Possible side effects include nausea, diarrhea, hair loss, fatigue, hypertension (high blood pressure), effects on bone marrow resulting in leukopenia (low white blood cell counts) and lymphopenia (low lymphocyte counts). 1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
