
Because each cancer type is different in origin, composition, and responsiveness to treatment, reliable prevention techniques are very difficult to identify. Evidence that an activity or dietary item prevents cancer is difficult to confirm because the goal of cancer prevention is to produce an outcome where nothing changes (i.e. cancer does not develop). Additionally, because cancer prevention cannot usually be accomplished by a single event, preventative measures must be taken for many years to give results that can be examned. Even if something is shown to help prevent a certain type of cancer, there is no guarantee that eating or behaving in a certain way will absolutely assure freedom from cancer development.
Much of the evidence for cancer prevention is not definitive. As an example, a diet low in fat 1 2 but high in fiber 3 4 , fruits and vegetables 5 6 has been associated with lower risks for several cancers. There have also been a number of studies that have shown no connection between these kinds of diets and reduced cancer cases 7 8 9 . Exercise has been shown to reduce cancer occurrence in some studies; in others, exercise does not seem to make a difference 10 11 .
Despite the conflicting evidence, the National Cancer Institute contends that the best way to help prevent cancer is to exercise, as well as eat a low calorie diet containing fiber, fruits, and vegetables. They also suggest that people should avoid a sedentary lifestyle, animal fats, and grilled meats to lessen the risk of developing cancer 12 . Research suggests that a combination of different essential nutrients is better than consuming a large amount of a single item. 13
Another way to help prevent cancer is to avoid behaviors that are generally accepted to increase your risk of cancer. Some of the behaviors linked to cancer development are tobacco use 14 15 16 , alcohol consumption 17 18 , obesity 17 19 20 , and sun exposure. 21 22 A study on Swedish families shows that an increased risk of cancer is more associated with environmental factors, diet and exercise rather than heredity. 23 The American Cancer Society suggests that a third of all American cancer deaths are linked to poor diet and lack of exercise. Additionally, another third of all cancer deaths are preventable by avoiding tobacco products. 24 The World Health Organization believes that 40% of cancer deaths world-wide could be prevented with proper diet, exercise, and tobacco avoidance. 25
View a 2015-2016 American Cancer Society publication on cancer prevention statistics.
In addition to these general guidelines, there are several specific compounds that have exhibited evidence that they may contribute to cancer prevention. 26
The following cancer prevention methods are described below in more detail.
- Exercise
- Antioxidants
- Plant Products (Phytochemicals)
- Drugs
- Cancer Vaccines
- Cancer Prevention Tables
If you find the material useful, please consider linking to our website
Exercise and Cancer Risk
- What is physical activity?
- Epidemiology of activity and cancer prevention.
- How does exercise reduce cancer risk?
- Racial differences in exercise-cancer risk reduction
Physical activity is defined as using skeletal muscles to exert more energy than simply resting in place.27
Physical activity takes many forms, from a daily stroll to heavy weightlifting. The majority of international guidelines recommend 150 minutes a week of moderate to vigorous physical exercise for a healthy adult. Preferably, exercise should be spread out throughout the week Research suggests that a range of health benefits can be achieved from being more physically active.28
Exercise can cause many changes in the human body. The types of changes depend on the amount and type of exercise. Exercise increases the amount of blood pumped by the heart (called cardiac output). This, in turn, leads to increased oxygen and blood flow. The increased heart rate also triggers the release of hormones. The adrenal glands, responsible for the release of epinephrine (adrenaline), and norepinephrine (noradrenaline), get activated. Exercise results in temporary elevation in lactate (produced by the breakdown of sugar in muscles), increased blood pressure, elevated blood glucose levels, and enhanced immune function. Exercise also speeds up metabolism and glucose consumption. Taken together, the effects of exercise are thought to reduce cancer risk.29
Epidemiology of Cancer and Exercise
Physical activity is strongly associated with lower risk of cancer at seven sites: bladder, colon, breast, endometrial, lung, esophageal, kidney, and stomach gastric. Physical activity has also been linked to reductions in pancreatic and ovarian cancer.30
Examples of the links between exercise and specific cancers include:
Bladder Cancer
Physical activity has been linked to reducing the risk of bladder cancer. Further research on outside factors must be made to confirm the association.31
Breast Cancer
Women with a family history of breast cancer, who engage in regular exercise have a reduced risk of postmenopausal breast cancer. The impact of exercise on the risk of premenopausal cancer is currently being studied.32
Colon Cancer
Physical activity has been shown to reduce the risk of colon cancer, especially among people who are overweight or obese.33
Endometrial Cancer
Although further studies are needed to measure the level of risk reduction and specific type of physical activity, moderate exercise is associated with reduced risk for endometrial cancer.34
Esophageal Cancer
Although existing studies indicate that physical activity offers protection against esophageal and gastric cancer, further research must be done to assess the intensity of the exercise needed to obtain the benefit.35
Kidney (Renal) Cancer
An older, 2013, review of the existing research came to the conclusion that physical activity may decrease the risk of kidney cancer by up to 22%. Research to determine the intensity, frequency, and duration of exercise needed to reduce kidney cancer is ongoing.36
How Does Exercise Reduce Cancer Risk?
Although the association between exercise and reduced cancer risk is clear, many gaps remain in our understanding of how exercise reduces cancer risk. So far, research has identified mostly changes in pathways critical to the to the development of cancer. Much like increased cholesterol is an indicator of possible heart disease, a number of ‘biomarkers’ have been found that link exercise to reduced cancer risk.37
Exercise lowers sex hormone levels.
Estrogen is a female sex hormone that maintains the female reproductive system. While estrogen is essential for the female body, women with elevated levels of estrogen and other sex hormones (androgens) are at a higher risk for developing breast cancer (7). Research has shown that exercise of sufficient frequency and intensity causes significant decreases in levels of these hormones.38
Exercise prevents high insulin levels
Insulin resistance leads to elevated blood levels of insulin, as well as elevated amounts of glucose in the blood stream. Insulin increases the chances of tumor development by activating cell proliferation, or cells dividing rapidly, and the inhibition of apoptosis, or programmed cell death.39 Most research investigating the effects on exercise on cancer growth focuses on cancers relationship to circulating metabolic factors, especially the insulin–glucose axis. Exercise is shown to have a correlation decrease in insulin levels of cancer patients40 41
Exercise reduces inflammation and regulates insulin levels42
In a study measuring the amount, type, and intensity of physical activity, and adiposity and diet, researchers found that aerobic and resistance training in combination were associated with decreases in cancer biomarkers.43
Biomarkers of inflammation include C reactive protein (CRP), a protein that increases in the blood during periods of inflammation and the immune system regulating proteins interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα).
Biomarkers of elevated insulin levels include c peptide, a byproduct of insulin production. Markers of insulin resistance includes high triglycerides (TG) and low high density lipoprotein cholesterol (TG/HDL) ratio, and adiponectin, decreased levels of adiponectin play a role in the development of obesity-related diseases.
Favorable biomarker levels for inflammation and insulin were tied to physical activity. Evidence favors moderately intensity exercise. Exercise that combines aerobic and resistance training have a stronger impact than vigorous activity or aerobic exercise alone.
The authors found that high physical activity, preferably aerobic plus resistance training, was associated with favorable biomarkers of inflammation and insulin response. Physical activity had similar associations for people with different levels of body fat (adiposity) and different diets. The best results were achieved by attaining high levels of all three. Physical activity reduces adiposity, essential in the relationship between the biomarkers of inflammation in the insulin responses.
Exercise Improves the immune system
Exercise promotes the activation of natural killer (NK) cells. These cells have the ability to recognize and kill other cells, including cancer cells. The increase of catecholamines, a hormone released during exercise, leads to increased numbers of NK cells in the blood stream.
Human NK cells are efficient cancer killers. The activation of these cells by exercise, plays a unique role in cancer therapy. Research suggests that exercise can increase the immune response to tumors. The activity can be further enhanced by the use of immune checkpoint inhibitors.44
Learn more about immune checkpoint inhibitor cancer treatments.
Exercise changes microRNA (miRNA) levels
RNA is one of the main information carriers in our cells. While DNA serves as the ‘storage’ form of our genetic information, RNA is the ‘working’ form. Tiny strings of RNA called microRNAs are known to regulate how many biological pathways work. Regular weekly exercise has been shown to change the amounts of different microRNAs in the blood. Blood fluid (sera) from people who had exercised was shown to reduce the survival of breast cancer cells in the laboratory. The researchers attribute the change to the microRNAs in those samples.45
Exercise Changes Oxygen Radical Levels and Signaling
Radicals are small chemicals produced in cells. The can be formed when cells capture engery from food, due to exposure to radiation or in other ways. They are very reactive and easily combine with - and damage - many important parts of cellls. Many of the important radicals in cells are derived from oxygen and are therefore called oxygen radicals.
The development of cancer can be driven by mutations and other cellular damage caused by radicals. Reactive oxygen and nitrogen radical levels in skeletal muscles (RONS) are increased by moderate-to-high intensity level exercise. At the same time, the increased levels lead to the activation of genes and proteins that block/destroy radicals. The boost in radical-destroying capability can slow or kill cancer cells. The combination of exercise with radiotherapy and/or chemotherapy may create an additive effect, increasing the effectiveness of the treatments at killing cancer cells.46
Skeletal muscles are thought to play an essential role in tying exercise to the reduction of cancer. Skeletal muscles produce signaling molecules (cytokines) and growth factors. These factors can travel in blood and effect the entire body. Some muscle products can inhibit tumor cells. They regulate the production of proteins that block cell reproduction, alter oxygen radical levels and lead to the release of signaling miRNAs into circulation.
A combination of aerobic and resistance appears to produce the best benefit for cancer patients and survivors.
Studies on the link between cancer and exercise are difficult to design. Because of this the results are not consistent. It is difficult to pinpoint the exact way that exercises reduces cancer risk. Studies that measure exercise and cancer tend to rely on self-reports of exercise. These are subjective and lack reliability and validity. Despite these limitations, our knowledge about the relationship between cancer risk and exercise is advancing.47
Racial Disparities In Physical Activity and Cancer Prevention
Race, ethnicity, and gender play a role in differences of physical activity in adults. Low financial resources can result in poor health in children, increasing the likelihood of chronic conditions and time of hospitalization. Economic status also impacts likely educational attainment. Poor childhood health plays a role in the transfer of economic status and healthy wellbeing across generations. 33.8% of White adults, 23.8% of Hispanic adults and 23.2% of Black adults engage in regular leisure physical activity. Non-work related physical activity is significantly lower among Black, Hispanic, and other minority racial groups, and these diferences can partially be explained by education level, socioeconomic status, and the locations in which the people live. In these groups, work-associated physical activity (blue collar labor), significantly outweighs non-work leisure physical activity. The absence of non-work physical activity lowers the benefits associated with exercise, including the prevention of cancer.48
One example can be found in breast cancer patients. A study of 1735 breast cancer patients, African American patients were found to be less likely to exercise the recommended amount - both before and after diagnosis.49
Antioxidants and Cancer
The possible role of antioxidants in the prevention and treatment of a variety of medical conditions has been very highly publicized. For some diseases, anti-oxidants may well play an important role. Unfortunately, some of the excitement is not based on scientific evidence. In order to understand how antioxidants work, it is first important to understand the process of oxidation.
What is Oxidation?
Oxidation is a chemical process. Oxidation is the reason that metals rust and apples turn brown. When this same process happens inside your body, it can harm cells and tissues. Free radicals are small chemicals that are responsible for oxidative damage. Free radicals can be contained in (or caused to form by) a variety of things including tobacco smoke, radiation (like sunlight or x-rays) and even the normal functioning of the human body. Free radicals are unstable and they can interact with, and alter, other molecules. Targets of free radicals include DNA, lipids and proteins. When free radicals interact with other molecules, they can cause changes (called oxidation) that interfere with the normal activity of the altered target molecules. The altered activity of the affected cell parts can cause severe problems for the cell and ultimately the entire body. You can learn more about oxidation in the Closer Look on this page.
What are Antioxidants?
Antioxidants are molecules that, as the name suggests, prevent or reverse oxidation. Examples of dietary antioxidants include vitamins C and E. Antioxidants are able to interact with and neutralize free radicals in vitro 50
51
, improve health and prolong life in animals 52
53
, and many studies are underway to investigate the potential of these compounds to prevent cancer in humans. Antioxidants are important to humans because they stop 'radicalized' molecules before they cause damage. Compounds that exhibit antioxidant properties do this by donating an electron to a free radical without needing to steal another electron. Unlike most compounds, antioxidants are stable with or without the electron they donate. Some antioxidants function in a "suicidal" manner, neutralizing free radicals by forming permanent bonds with them.
Sources and Uses of Antioxidants
There are many good sources of antioxidants including green tea, berries, tomatoes and soy. Antioxidants can be found in many fruits and vegetables because plants produce antioxidants to help protect themselves from free radicals created by radiation from the sun. The body naturally produces some antioxidants that help protect against free radical damage. Unfortunately, these do not provide complete protection. Humans must consume antioxidants from other sources if they wish to decrease their risk of developing diseases that are increased by free radical damage 54
. Outside of the body, antioxidants can also be used for many practical purposes: museum curators use them to preserve artifacts derived from living things, they can prevent food from spoiling. They are also used to make better rubbers, plastics, automobile fuels and paint 55
.
NOTE: Just because something is healthy does not mean more is better. Even the healthiest substances can cause harm if taken in large amounts. In fact, there has been some recent evidence linking antioxidants to the spread of cancer. The benefits of antioxidants aren't limited to healthy cells; it seems they can support the survival and spread of cancer cells too. In one study, mice were given an anti-diabetic drug that activated a protein, called NRF2, in their cells. This protein increases the levels of antioxidants in cells. NRF2 activation seemed to increase cell migration and metastasis in mice with pre-existing tumors. Be sure to tell your physician about any drugs you may be taking, and always ask before starting any supplements. 56
Watch the full interview about antioxidants with urologist Dr. John Petros.
A Closer Look at Free Radicals
Atoms are composed of a nucleus, containing particles called protons and neutrons, and a group of particles (electrons) that constantly circle the nucleus like satellites around the earth. In most molecules electrons travel in pairs around the nucleus.Free radicals are an exception. At least one atom in a free radical has a single (unpaired) electron circling the nucleus. This single electron gives the atom a charge, making it very attracted to other molecules. A molecule with an unpaired electron is said to be 'radicalized'. These radicalized compounds, or free radicals, can quickly react with other molecules. For this reason these compounds are also called reactive species. Oxygen is the most common reactive species found in the human body and when it acquires an extra electron it is called a reactive oxygen species (ROS).
Free radicals 'steal' an electron from a nearby molecule so that all of their electrons are in pairs. The affected target molecule would then become a radical. A chain reaction of electron 'theft' can occur within a cell. Free radicals can affect just about any structure in a cell, including DNA. If free radicals steal an electron from DNA, the genetic code can be damaged and cell function damaged. DNA damage caused by free radicals has been associated with aging, rheumatoid arthritis, inflammatory bowel disease, acute respiratory distress syndrome (ARDS), emphysema, and some types of cancer. 57 55 58
Plant Products (Phytochemicals) and Cancer
Phytochemicals are compounds found in plants. The phytochemicals below are being investigated to see if they are beneficial to human health; even when extracted from the plant from which they originate. In recent years, there has been a large amount of research to investigate the usefulness of specific natural compounds in the prevention and treatment of cancer. The list below is not meant to be complete. There are numerous other chemicals that are being examined. We have chosen some of the best-studied and most widely publicized agents:
Choosing an item from the list below will take you to the Plant Products page in our Integrative Oncology section.
- Anthocyanin (Berries)
- Curcumin (Turmeric, Curry)
- EGCG (Green Tea)
- Lycopene (Tomatoes)
- Phytoestrogens (Soy)
- Pycnogenol (Pine Trees)
- Resveratrol (Grapes, Wine)
- Selenium (Nuts)
Drugs
Drugs that are used to help prevent cancer are highly regulated by the federal government to insure quality and safety. Most drugs that are suggested by doctors to help prevent cancer are for a specific population that is at a high risk of developing certain cancer types. These drugs are not suggested for all people because they can cause other problems that may not be worth the cost of protection. Nevertheless, some drugs have been shown to decrease the risk for cancer and have been approved by the US Food and Drug Administration for cancer prevention. These include non-steroidal anti-inflammatory drugs (NSAIDs) and the hormonal antagonists tamoxifen and raloxifene, discussed in more detail below.
Non-steroidal Anti-inflammatory Drugs (NSAIDs)

Types of NSAIDs
COX-1 Inhibitors:
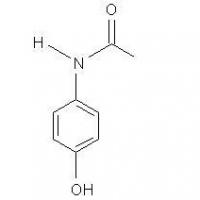
Structure of Acetaminophen
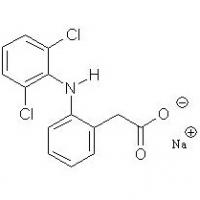
Structure of Diclofenac
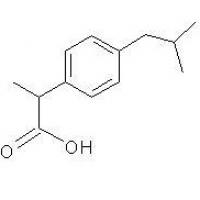
Structure of Ibuprofen
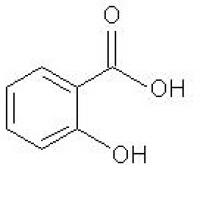
Structure of Salicylic Acid
COX-2 Inhibitors:
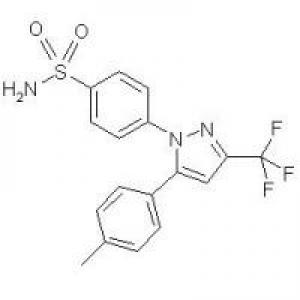
Structure of Celecoxib
Structure of Naproxen
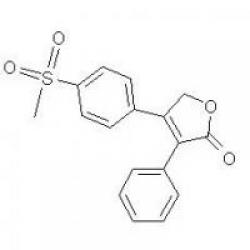
Structure of Rofecoxib
Structure of Valdecoxib
Intro and Background
The inflammatory response is a normal function of the human immune system. This process helps repair the body after injury. When you get cut or hurt, the area usually becomes red, hot, and swollen. In part, this is caused by the immune system as it works to heal the damaged area of your body. This response brings nutrient rich blood to the area so injured tissues can be repaired and new cells can grow 59
. Despite its normally positive function, research has shown that the human inflammatory response is important in controlling the environment of cancer cells. Inflammation can promote tumor angiogenesis, initiation, growth and metastasis. Cells of the immune system, such as macrophages, may also be negatively affected by inflammation. 60
61
62
63
. Also, the same cells that are stimulated by the immune response to grow and replicate may malfunction and grow without proper regulation; this behavior may lead to the initiation of a cancerous growth. 59
.
Learn MORE about angiogenesis and metastasis
Non-steroidal anti-inflammatory drugs interfere with the activity of a family of enzymes called cyclooxygenases (COX). COX inhibitors prevent the enzymes from producing chemical signals responsible for inflammation, pain and possibly tumor growth 64 . There are two types of NSAIDs, grouped by the form of COX they inhibit (COX-1 or COX-2). NSAIDs are quite common and are commonly known as pain relievers [acetaminophen (Tylenol®), diclofenac (Voltaren®), ibuprofen (Advil® ), salicylic acid (Aspirin®), celecoxib (Celebrex®), naproxen (Aleve®), rofecoxib (Vioxx®), and valdecoxib (Bextra®)]. Aspirin is also taken for the prevention of heart disease. For this use, the average, safest, and most effective dose of aspirin usually recommended by doctors is 81 mg/day 65 .
Scientific Research
Research suggests that NSAIDs are effective chemopreventative agents but significant negative effects have also been identified. Regular use of aspirin has been shown to reduce the risk of colorectal cancer and recurrent colorectal cancer in humans 66
67
64
68
69
70
71
. Exactly how these drugs reduce cancer risk is still unclear but seems to be linked to their ability to block the cyclooxygenase (COX) enzymes.72
COX-1 inhibitors are associated with digestive problems such as excessive bleeding and ulcers.65
COX-2 inhibitors have recently come under scrutiny because they were identified as increasing the risk of heart attacks and strokes 73 . Because of the risks associated with NSAIDs, only people at high risk of cancer are typically given these drugs as a preventative measure. Researchers are now working to identify the sub-population of people who should take NSAIDS to lower their risk of colon cancer. 74
Aspirin, a type of NSAID, has been documented in some cases to reduce the risk of certain cancers, notably colorectal cancer, and potentially cancers of the oesophagus, stomach, breast, ovary, and lung. Aspirin has also been shown to reduce the risk of Barrett's esophagus, a precursor to esophageal cancer.75 . Though daily aspirin use has been shown to decrease cancer mortality,76 aspirin is nevertheless a drug, and long-term use may lead to other problems. Aspirin has even proposed to increase risk for kidney cancer 77
Studies have shown that individuals with an increased risk of colorectal cancer may be able to reduce recurrence of adenomas or advanced adenomas in the colon with the use of aspirin 78 . However, its effectiveness in prevention may be dependent on its duration of use and the dose taken.79 80 The long term use of aspirin has been documented to reduce the incidence of colorectal cancer. Long-term use was even associated with decreased abnormal methylation of genes related to cancer. 81 In contrast, studies with smaller, short term usage have not yielded these results.82 83 The US Preventative Services Task Force has concluded, though, that overall, taking aspirin to reduce the risk for colorectal cancer may be doing more harm than good for individuals with an average risk of developing colorectal cancer.84 More research is needed to determine if the benefits outweight the possible risks for people with an increased risk of colorectal cancer. In summary, aspirin has been shown to reduce the risk of developing colorectal cancer and adenomas, and reduce the chance of recurrence of colorectal cancer and adenomas in individuals with a history of colorectal cancer. However, taking aspirin for colorectal cancer prevention depends on an individual's risk of developing cancer; the possible negative effects of aspirin need to be weighed against its chemoprevention benefits.
Results of studies on the ability of aspirin to reduce the risk of prostate cancer have been inconsistent.85 Some research indicates that aspirin may be able to reduce the level of prostate specific antigen (PSA) in individuals with latent cancer, and thus affect the detection of prostate cancer.86 Other research indicates that, as with colorectal cancer, the dosage and duration of aspirin intake may play a role in its protective effect 87 . For example, researchers found that exposure to an average dose of at least 80 mg of aspirin for 8 years led to a decline of 18% in prostate cancer risk. However, one year after ending the 7 year regular aspirin intake, no protective effect was found.
It is unclear whether aspirin reduces the risk of breast cancer. Some studies have found that aspirin use is associated with reduced risk for breast cancer 88 , but others have found no significant reduction in risk with use of aspirin 87 .It is possible that the effects are different for different types of breast cancer. For example, daily intake of aspirin was associated with reduction in risk for ER-positive breast cancer 87 . Recent research also indicates that aspirin may prevent the metastasis of breast cancer 89 .
Low-dose aspirin may help reduce the risk of ovarian cancer, and could improve survival of women with ovarian cancer. By looking at information gathered by 13 different studies, involving 750,000 women, researchers showed that daily aspirin use reduced risk of ovarian cancer by 10%.90 A separate study, using information from the Nurse's Health Studies, found that daily aspirin users had up to a 30% improvement in their survival.91
A large clinical trial, ASPirin in Reducing Events in the Elderly (ASPREE) studied the impact of daily low dose (100mg) aspirin on the health outcomes of elderly people. The study followed over 19,000 Australians and Americans aged 70 yrs and higher (65+ years for American minority participants). The median time that participants were followed was 4.7 years. The study found no statistically significant differences in the number of cancer cases that arose but that the people taking aspirin were more likely to have advanced cancer (cancer that had spread). Taking aspirin was also linked to increased death from some cancers. The results indicate that aspirin may affect the elderly differently than younger people.92
US Food and Drug Administration Approval
Celecoxib (Celebrex®) is the only NSAID that has been approved to treat cancer. It is used to reduce polyps for people with a rare genetic disorder (adenomatous polyposis)93
. In 2007, the US Preventive Services Task Force recommended against the use of aspirin for the prevention of colorectal cancer.94
The recommendation was the same in 2013 review.95
Hormonal Antagonists
Drugs:
Tamoxifen- Nolvadex®
Raloxifene- Evista®, Keoxifene, Raloxifene Hydrochloride
Classified as:
Hormonal Antagonists
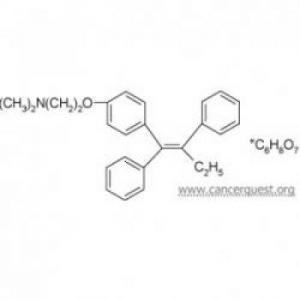
Structure of Tamoxifen
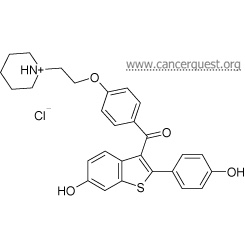
Structure of Raloxifene
Intro and Background
Tamoxifen and Raloxifene are both prescription drugs that are structurally similar to the hormone estrogen. Tamoxifen was originally developed in the 1960s as a possible contraceptive. After it was found to be inneffective for this use, doctors began researching it as a way to fight estrogen stimulated cancers. 96
97
In the 1970s, it was tested on late stage breast cancer and was found to be a potent treatment. In 1998, it was approved as the first chemopreventative agent for women with a high risk of breast cancer. 97
98
Tamoxifen and Raloxifene are classified as selective estrogen receptor modulators (SERMs) because they are able to block a cell's response to estrogen. Tamoxifen is also known to trigger uterine cancer and blood clots that may block blood vessels (thromboemboli). Because of these negative side-effects, doctors suggest that only women with a high risk of breast cancer should take the drug as a preventative measure 98
99
.
Scientific Research
Early studies showed that breast cancer tumorogenesis was inhibited in rats treated with tamoxifen 100
. In addition to the positive effects of tamoxifen, it has been found to increase the risk of endometrial and uterine cancers. This may be due to its ability to damage DNA in healthy cells 101
. Both tamoxifen and raloxifene have both been shown to reduce the incidence of invasive breast cancer by as much as fifty percent in high risk women.102
103
104
105
US Food and Drug Administration Approval
Tamoxifen was approved by the FDA for breast cancer treatment in 1977. Tamoxifen was subsequently approved as a way to help prevent surgically removed breast cancer from reoccurring as well to help prevent breast cancer in high risk women 97
. Raloxifene has been approved by the FDA to help prevent and combat cancer and bone loss (osteoporosis). 106
103
Cancer Vaccines
The prevention of cancer is the ultimate goal of cancer researchers and clinicians. One good way to accomplish this is to prevent infection with agents (viruses, bacteria, and parasites) known to cause cancer. Vaccines have been developed and approved to prevent infection with hepatitis B virus, a cause of liver cancer, and the human papillomavirus, the major cause of cervical cancer and a cause of cancers of the head/neck and urogenital tract of men and women. Learn more about the approved vaccines:
Cervical Cancer Vaccines
The development of vaccines against HPV is a major step in the fight against cervical cancer.
Currently, there are three vaccines approved for the prevention of infection with HPV, Gardasil®, Gardasil 9® and Cervarix®.
Gardasil® is FDA approved for the prevention of HPV types 6, 11, 16 and 18 infection in young women aged 9-26. On October 16, 2009, the FDA also approved Gardasil® to prevent genital warts caused by HPV types 6 and 11 in young men aged 9-26.107 108 In 2016, the CDC changed their recommendation for 11- to 12-year-olds, allowing them to take only two doses of HPV vaccine instead of three doses. People vaccinated at 15 through 26 years of age, still need to get three doses of of the vaccine.109
Cervarix® also prevents infection by HPV 16 and 18. Cervarix® was approved for use in the United States in October 2009.110
Importantly, the vaccinations are prophylactic and are not effective against the progression of pre-existing HPV infection or cervical dysplasia, and should not deter women from annual screening tests, especially since not all cancer causing (oncogenic) forms of HPV are included in the vaccines.110 111
Watch the video to learn more about HPV vaccines. Watch the full interview with Dr. Ira Horowitz.
Because of exciting recent developments in this field, we will cover this treatment in more depth than some others and will discuss the development of the treatment
The majority of cervical cancer is believed to be caused by the Human Papillomavirus (HPV). There are more than 100 variants (subtypes) of HPV but only a small subset are associated with human cancer. HPV subtypes 16 and 18 are the variants most commonly associated with human cervical cancer. Several characteristics of HPV make it a good target for vaccine development. The virus is simple, small, and has a stable genome. HPV vaccine development and clinical trials are currently underway.
Gardasil®
The Strategy
As discussed in the previous sections, the goal of vaccines is to increase the response of the immune system to particular antigens. The idea is that if our immune system 'sees' the protein again, it will respond very strongly. In the case of viruses, the immune system often reacts to the proteins on the outside of the virus particle.
In a laboratory, it is possible to construct non-infectious virus-like particles (VLPs) that are similar to the infectious virus but are free of viral DNA and can therefore not reproduce. These VLPs contain viral proteins and are capable of generating the same natural humoral immune response following injection into the body. Two different VLP-based Human Papilloma Virus vaccines are at advanced stages of development/usage.
The Agent
Gardasil®
The first preventative cancer vaccine to receive FDA approval was Gardasil®. Gardasil® was designed to prevent infection by four different subtypes of HPV (6, 11, 16, and 18). HPV types 6 and 11 together, cause 90% of the cases of genital warts while 16 and 18 combined, are responsible for 70% of cervical cancer cases. Gardasil® contains VLPs containing the capsid protein of each of these four strains of HPV.
Gardasil® is produced by Merck Pharmaceuticals. The agent was approved by the FDA on June 8, 2006 for the prevention of cervical cancer (and genital warts) in females 9-26 years old and is being tested for its effectiveness in other age groups and in combination with other vaccines. On October 16th,2009 Gardasil® was approved for use in boys and men 9-26 years of age.
The Data
Following positive phase I and II clinical trials, a total of 12,167 women, aged 16-26 years, enrolled in a phase III trial at 90 different study centers in Brazil, Colombia, Denmark, Finland, Iceland, Mexico, Norway, Peru, Poland, Singapore, Sweden, the United Kingdom and the United States. Of the more than 12,000 participants, 6,082 females received a three dose regimen of Gardasil while the remaining 6,075 received a placebo. The study evaluated the occurrence of HPV 16/18 associated cervical pre-cancers and non-invasive cancers.
Specifically, participants were screened for moderate (2) and high (3) grade cervical intraepithelial neoplasia (CIN), Grade 3 CIN is also known as carcinoma in situ (CIS). CIS is an immediate precursor to invasive squamous cell cervical cancer. Participants were also examined for the occurrence of AIS adenocarcinoma in situ (AIS), a precursor to glandular cervical cancer. The results of the trial showed that Gardasil® prevented 100 percent of high-grade pre-cancer and non-invasive cancers associated with HPV 16/18. Gardasil® was also found to reduce the risk of developing high-grade pre-cancer and non-invasive cancer in women who may have violated the protocol or become infected with the virus during the time of the trial. The treatment received FDA approval in the summer of 2006.112 113 114
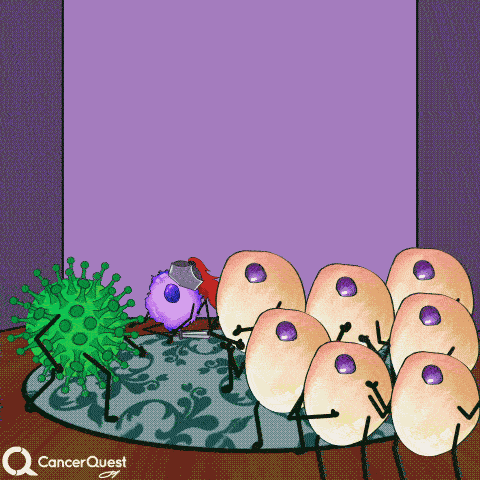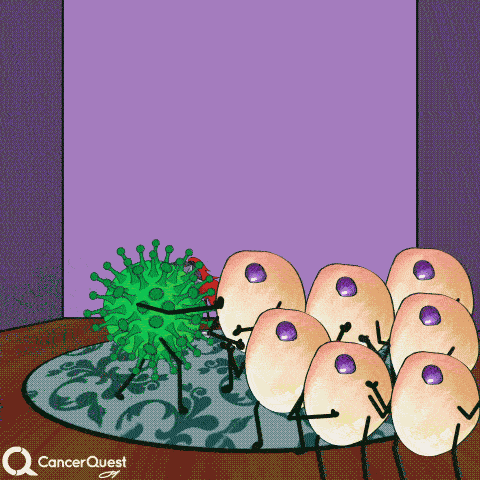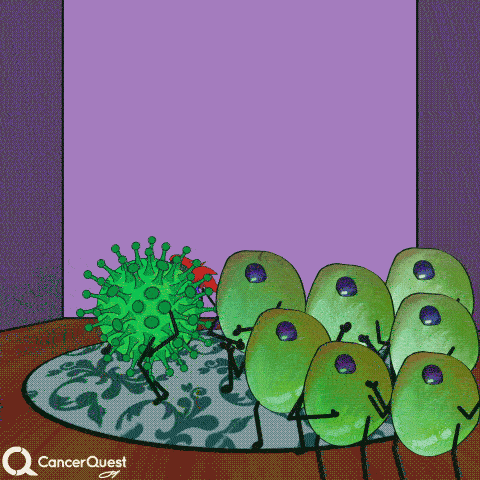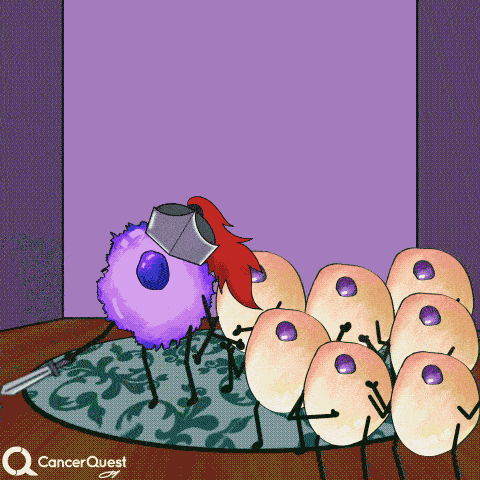
In most cases, infections with HPV are cleared entirely by the immune system. Cancer does not form.
If infections last a long time, often many years, cancer can develop.
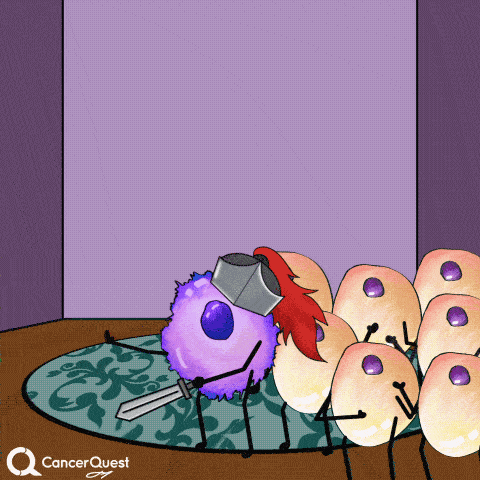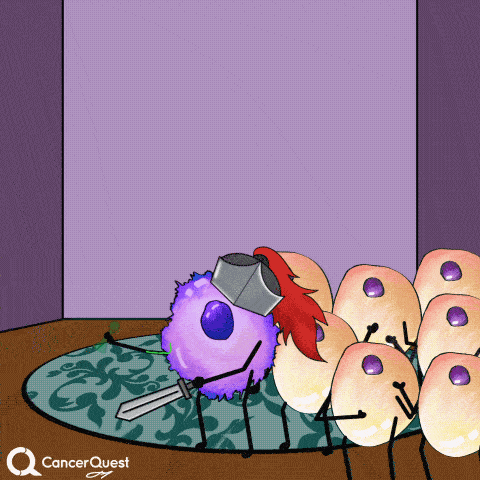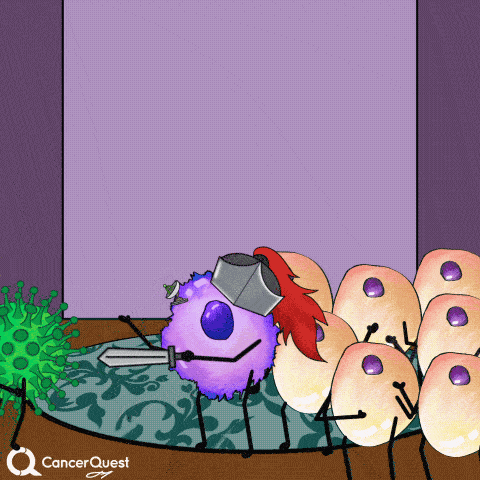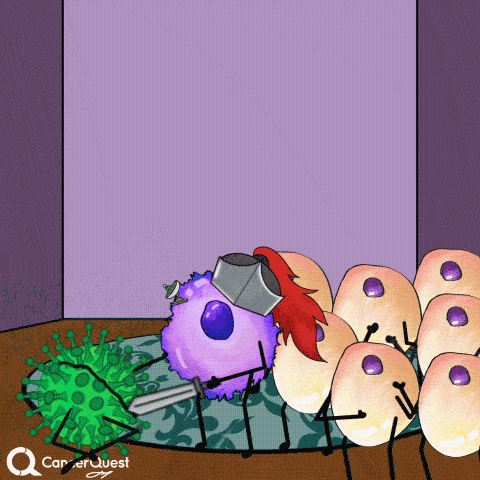
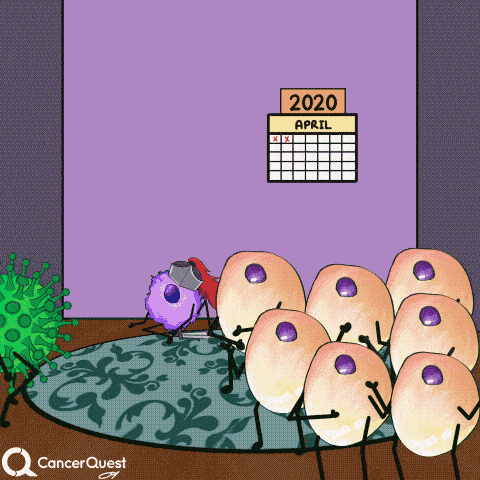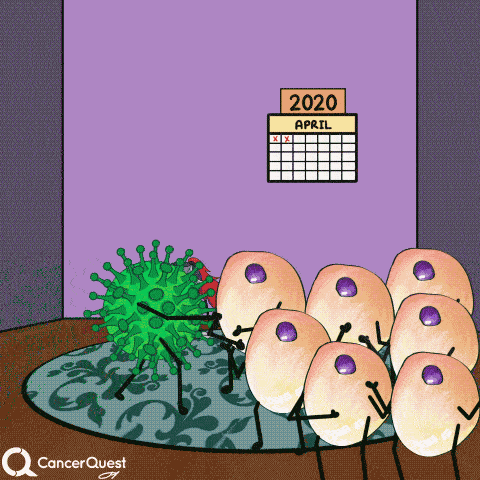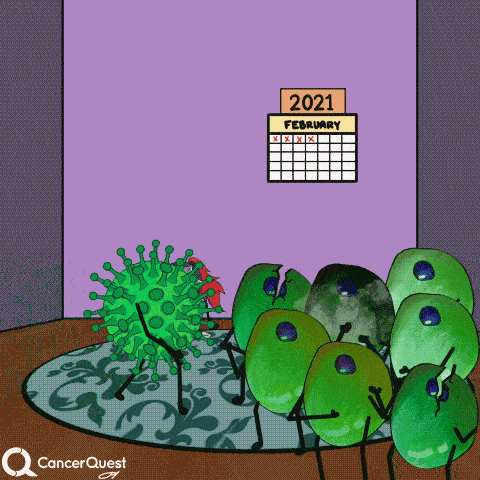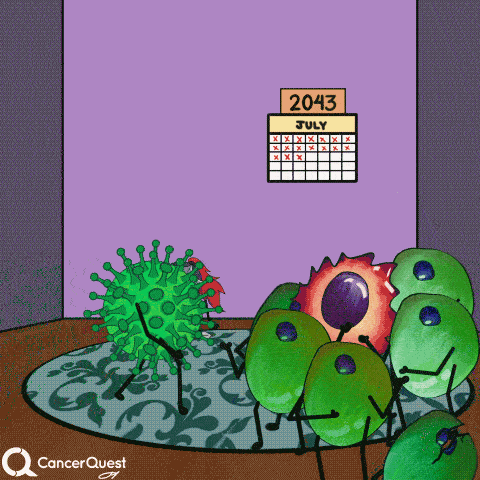
Vaccines give the immune system a chance to get prepared, so when the actual virus comes in, it can be eliminated quickly.
This prevents infection and the chance of cancer.
Gardasil 9®
Building on the success of Gardasil®, a new vaccine was developed which protects against additional forms of the human papillomavirus.
Gardasil 9® covers 9 HPV types, 4 of which are covered in the previously-approved Gardasil® and 5 of which are not covered by the previously-approved Gardasil®. The 5 additional HPV types are 31, 33, 45, 52, and 58, which are responsible for approximately 20% of cervical cancers. Gardasil®9 is administered as 3 separate shots: initial dose followed by a second dose 2 months after the initial dose and a third dose 6 months after the initial dose.
In a clinical study conducted in the United States and overseas, approximately 14,000 participants (females ages 16 to 26) were either given Gardasil® or Gardasil®9. Gardasil®9 was found to be 97% effective at preventing the diseases caused by HPV types 31, 33, 45, 52, and 58. It was also found to be as effective as Gardasil® at preventing the diseases caused by HPV types 6, 11, 16, and 18. Commonly reported side effects include injection site pain, swelling, redness, and headache.
Gardasil 9® is produced by Merck Pharmaceuticals. On December 10th of 2014, Gardasil 9® was approved for use in females 9-26 years of age and in males 9-15 years of age. In 2015, the vaccine was approved for males 9-15 years of age, and in 2016, a two-dose regimen was approved for individuals aged 9-14115
More on Gardasil® from Merck
Search for clinical trials involving Gardasil® at the NCI
Cervarix®
A second HPV vaccine, Cervarix®, produced by GlaxoSmithKline, was approved by the U.S. Food and Drug Administration (FDA) in October 2009 for use in girls age 10 to 25.116 The vaccine is designed to protect against infection by HPV types 16 and 18, these two subtypes of HPV are responsible for 70% of cervical cancer cases. In addition to two VLPs, the vaccine also contains chemicals (aluminum hydroxide and 3-deacylated monophosphoryl lipid A (AS04)) designed to increase the immune response to the viral proteins.
A Phase III double-blind, placebo-controlled trial of Cervarix® was conducted with more than 1,100 women in North America and Brazil between the ages of 15 and 25. Those receiving Cervarix® received three doses of the vaccine over a six month period, similar to the manner in which Gardasil® is administered. Follow-up was conducted for 27 months. Researchers found a 92 percent efficacy rate against new infection and 100 percent protection against persistent HPV infection. The results also showed that AS04 helped the vaccine to elicit a stronger antibody response than would occur with natural infection.117
In 2016, Cervarix® was withdrawn from the U.S. market118
Liver Cancer (Hepatitis Virus)
Chronic infection with hepatitis viruses is a major risk factor for the development of liver cancer. Both hepatitis B virus (HBV) and hepatitis C virus (HCV) are associated with liver cancer.119 120 Gobally, infection with hepatitis B virus is extremely common, with an estimated 240 million people infected worldwide in 2005.121
A vaccine against hepatitis B virus was approved in 1981, making it the first cancer prevention vaccine.122 Today, babies in the US are routinely vaccinated against hepatitis B shortly after birth. It is also recommended that adults receive vaccinations against hepatitis B123 124 There is no vaccine available to prevent infection with hepatitis C.
Cancer Prevention Tables
| Classification |
Chemical |
| Drugs | NSAIDs, Tamoxifen, Raloxifene |
| Phytochemicals | Anthocyanin, Curcumin, EGCG, Lycopene, Phytoestrogens, Pycnogenol, Resveratrol, Selenium, Vitamin E |
| Biological Process Affected |
Chemical |
| Angiogenesis | Curcumin, EGCG, Resveratrol |
| Apoptosis | Anthocyanin, Curcumin, EGCG, Lycopene, Resveratrol, Selenium |
| Inflammation | Anthocyanin, Pycnogenol, NSAIDs |
| Metastasis | Curcumin, NSAIDs, Vitamin E, Resveratrol |
| Oxidation (i.e. Antioxidants) |
Anthocyanin, Curcumin, EGCG, Lycopene, Phytoestrogens, Pycnogenol |
| Proliferation | Anthocyanin, Curcumin, NSAIDs, EGCG, Selenium, |
| Enzyme Activity | NSAIDs |
| Hormone Activity | Tamoxifen, Raloxifene |
- 1Littman AJ, Beresford SA, White E: The association of dietary fat and plant foods with endometrial cancer (United States). Cancer Causes Control 12 (8): 691-702, 2001 [PUBMED]
- 2McCann SE, Freudenheim JL, Marshall JR, et al.: Diet in the epidemiology of endometrial cancer in western New York (United States). Cancer Causes Control 11 (10): 965-74, 2000. [PUBMED]
- 3Howe GR, Benito E, Castelleto R, et al.: Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst 84 (24): 1887-96, 1992. [PUBMED]
- 4Morse DE, Pendrys DG, Katz RV, et al.: Food group intake and the risk of oral epithelial dysplasia in a United States population. Cancer Causes Control 11 (8): 713-20, 2000. [PUBMED]
- 5Buiatti E, Palli D, Decarli A, et al.: A case-control study of gastric cancer and diet in Italy: II. Association with nutrients. Int J Cancer 45 (5): 896-901, 1990. [PUBMED]
- 6Neuhouser ML, Patterson RE, Thornquist MD, et al.: Fruits and vegetables are associated with lower lung cancer risk only in the placebo arm of the beta-carotene and retinol efficacy trial (CARET). Cancer Epidemiol Biomarkers Prev 12 (4): 350-8, 2003. [PUBMED]
- 7Fuchs CS, Giovannucci EL, Colditz GA, et al.: Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med 340 (3): 169-76, 1999 [PUBMED]
- 8Michels KB, Edward Giovannucci, Joshipura KJ, et al.: Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst (2000) 92(21): 1740-52. [PUBMED]
- 9Jain MG, Rohan TE, Howe GR, et al.: A cohort study of nutritional factors and endometrial cancer. Eur J Epidemiol (2000) 16 (10): 899-905. [PUBMED]
- 10Friedenreich CM: Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev 10 (4): 287-301, 2001. [PUBMED]
- 11Schouten LJ, Goldbohm RA, van den Brandt PA: Anthropometry, physical activity, and endometrial cancer risk: results from the Netherlands Cohort Study. J Natl Cancer Inst 96 (21): 1635-8, 2004. [PUBMED]
- 12Vainio H, Weiderpass E. Fruit and Vegetables in Cancer Prevention. Nutrition and Cancer. (2006) 54(1):111-42 [PUBMED]
- 13Greenwald P, Anderson D, Nelson SA, Taylor PR. Clinical trials of vitamin and mineral supplements for cancer prevention. Am J Clin Nutr. (2007) 85(1):314S-317S. [PUBMED]
- 14Boffetta P, Pershagen G, Jöckel KH, et al.: Cigar and pipe smoking and lung cancer risk: a multicenter study from Europe. J Natl Cancer Inst 91 (8): 697-701, 1999 [PUBMED]
- 15Anthonisen NR, Skeans MA, Wise RA, et al.: The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 142 (4): 233-9, 2005. [PUBMED]
- 16Koh HK: The end of the "tobacco and cancer" century. J Natl Cancer Inst 91 (8): 660-1, 1999. [PUBMED]
- 17 a b Morimoto LM, White E, Chen Z, et al.: Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control 13 (8): 741-51, 2002. [PUBMED]
- 18Bagnardi V, Blangiardo M, La Vecchia C, et al.: Alcohol consumption and the risk of cancer: a meta-analysis. Alcohol Res Health 25 (4): 263-70, 2001 [PUBMED]
- 19Purdie DM, Green AC: Epidemiology of endometrial cancer. Best Pract Res Clin Obstet Gynaecol 15 (3): 341-54, 2001 [PUBMED]
- 20Bergström A, Pisani P, Tenet V, et al.: Overweight as an avoidable cause of cancer in Europe. Int J Cancer 91 (3): 421-30, 2001. [PUBMED]
- 21Preston DS, Stern RS: Nonmelanoma cancers of the skin. N Engl J Med 327 (23): 1649-62, 1992 [PUBMED]
- 22English DR, Armstrong BK, Kricker A, et al.: Case-control study of sun exposure and squamous cell carcinoma of the skin. Int J Cancer 77 (3): 347-53, 1998. [PUBMED]
- 23Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish Family-Cancer Database. Int J Cancer 2002;99:260266 [PUBMED]
- 24Lawrence H. Kushi, Tim Byers, Colleen Doyle, Elisa V. Bandera, Marji McCullough, Ted Gansler, Kimberly S. Andrews, Michael J. Thun and The American Cancer Society 2006 Nutrition and Physical Activity Guidelines Advisory Committee. CA Cancer J Clin (2006) 56; 254-281 [PUBMED]
- 25World Health Organization. Cancer Fact Sheet No. 297. February 2006. Accessed 2 June 2010. [http://www.who.int/mediacentre/factsheets/fs297/en/index.html]
- 26Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortaily from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348: 1625-38 [PUBMED]
- 27World Health Organization "Physical Activity" https://www.who.int/health-topics/physical-activity#tab=tab_1
- 28. (2018). The Physical Activity Guidelines for Americans. Jama, 320(19), 2020-2028. http://doi.org/10.1001/jama.2018.14854 (Original work published December 2018) [PUBMED]
- 29. (2017). Exercise and cancer: from "healthy" to "therapeutic"?. Cancer Immunology, Immunotherapy : Cii, 66(5), 667-671. http://doi.org/10.1007/s00262-017-1985-z (Original work published May 2017) [PUBMED]
- 30. (2018). Physical activity and cancer: an umbrella review of the literature including 22 major anatomical sites and 770 000 cancer cases. British Journal Of Sports Medicine, 52(13), 826-833. http://doi.org/10.1136/bjsports-2017-098391 (Original work published July 2018) [PUBMED]
- 31. (2014). The association between physical activity and bladder cancer: systematic review and meta-analysis. British Journal Of Cancer, 110(7), 1862-70. http://doi.org/10.1038/bjc.2014.77 (Original work published April 2014) [PUBMED]
- 32. (2019). Adult Physical Activity and Breast Cancer Risk in Women with a Family History of Breast Cancer. Cancer Epidemiology, Biomarkers & Prevention : A Publication Of The American Association For Cancer Research, Cosponsored By The American Society Of Preventive Oncology, 28(1), 51-58. http://doi.org/10.1158/1055-9965.EPI-18-0674 (Original work published December 2019) [PUBMED]
- 33. (2018). Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: a systematic review and meta-analysis. Bmc Cancer, 18(1), 71. http://doi.org/10.1186/s12885-017-3970-5 (Original work published December 2018) [PUBMED]
- 34. (2007). Physical activity and endometrial cancer risk, a systematic review of current evidence. Cancer Epidemiology, Biomarkers & Prevention : A Publication Of The American Association For Cancer Research, Cosponsored By The American Society Of Preventive Oncology, 16(4), 639-48. (Original work published April 2007) [PUBMED]
- 35. (2014). Physical activity and risks of esophageal and gastric cancers: a meta-analysis. Plos One, 9(2), e88082. http://doi.org/10.1371/journal.pone.0088082 (Original work published December 2014) [PUBMED]
- 36. (2013). The association between physical activity and renal cancer: systematic review and meta-analysis. British Journal Of Cancer, 108(4), 798-811. http://doi.org/10.1038/bjc.2013.37 (Original work published March 2013) [PUBMED]
- 37. (2007). Exercise and biomarkers for cancer prevention studies. The Journal Of Nutrition, 137(1 Suppl), 161S-169S. http://doi.org/10.1093/jn/137.1.161S (Original work published December 2007) [PUBMED]
- 38. (2007). Exercise and biomarkers for cancer prevention studies. The Journal Of Nutrition, 137(1 Suppl), 161S-169S. http://doi.org/10.1093/jn/137.1.161S (Original work published December 2007) [PUBMED]
- 39. (2007). Exercise and biomarkers for cancer prevention studies. The Journal Of Nutrition, 137(1 Suppl), 161S-169S. http://doi.org/10.1093/jn/137.1.161S (Original work published December 2007) [PUBMED]
- 40. (2019). Association of type and intensity of physical activity with plasma biomarkers of inflammation and insulin response. International Journal Of Cancer, 145(2), 360-369. http://doi.org/10.1002/ijc.32111 (Original work published December 2019) [PUBMED]
- 41. (2013). Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, Behavior, And Immunity, 30 Suppl, S75-87. http://doi.org/10.1016/j.bbi.2012.05.001 (Original work published March 2013) [PUBMED]
- 42. (2013). Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, Behavior, And Immunity, 30 Suppl, S75-87. http://doi.org/10.1016/j.bbi.2012.05.001 (Original work published March 2013) [PUBMED]
- 43. (2019). Association of type and intensity of physical activity with plasma biomarkers of inflammation and insulin response. International Journal Of Cancer, 145(2), 360-369. http://doi.org/10.1002/ijc.32111 (Original work published December 2019) [PUBMED]
- 44. (2017). Exercise and cancer: from "healthy" to "therapeutic"?. Cancer Immunology, Immunotherapy : Cii, 66(5), 667-671. http://doi.org/10.1007/s00262-017-1985-z (Original work published May 2017) [PUBMED]
- 45. (2020). Anticancer effect of physical activity is mediated by modulation of extracellular microRNA in blood. Oncotarget, 11(22), 2106-2119. http://doi.org/10.18632/oncotarget.27609 (Original work published June 2020) [PUBMED]
- 46. (2020). Exercise shapes redox signaling in cancer. Redox Biology, 35, 101439. http://doi.org/10.1016/j.redox.2020.101439 (Original work published December 2020) [PUBMED]
- 47. (2013). Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain, Behavior, And Immunity, 30 Suppl, S75-87. http://doi.org/10.1016/j.bbi.2012.05.001 (Original work published March 2013) [PUBMED]
- 48. (2013). Racial, Ethnic, and Gender Differences in Physical Activity. Journal Of Human Capital, 7(4), 378-410. (Original work published December 2013) [PUBMED]
- 49. (2014). Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer, 120(14), 2174-82. http://doi.org/10.1002/cncr.28630 (Original work published July 2014) [PUBMED]
- 50Valcheva-Kuzmanova S.V., Belcheva A. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. Journal of Carcinogenesis (2007) Apr 18; 6: 4 [PUBMED]
- 51Lin Y.G., Kunnumakkara A., et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by targeting the Nuclear Factor-ºB Pathway. Clin Cancer Res 2007 13: 3423-3430 [PUBMED]
- 52Yanase S, Yasuda K, Ishii N: Adaptive responses to oxidative damage in three mutants of Caenorhabditis elegans (age-1, mev-1 and daf-16) that affect life span. Mech Ageing Dev. (2002) 123:1579-1587 [PUBMED]
- 53Missirlis F, Phillips JP, Jackle H: Cooperative action of antioxidant defense systems in Drosophila. Curr Biol. (2001) 11:1272-1277 [PUBMED]
- 54Free Radicals in Biology and Medicine Fifth Edition (2015) Barry Halliwell and John M. C. Gutteridge. Oxford University Press. Available online at https://global.oup.com/academic/product/free-radicals-in-biology-and-medicine-9780198717485
- 55 a b Halliwell, Barry. (May 3, 2005) Free radicals and other reactive species in disease. In: Encyclopedia of Life Sciences. John Wiley & Sons, Ltd: Chichester http://www.els.net/ [doi:10.1038/npg.els.0006101] [http://www.els.net/ [doi:10.1038/npg.els.0006101]]
- 56Wang, H., X. Liu, M. Long, Y. Huang, L. Zhang, R. Zhang, Y. Zheng, X. Liao, Y. Wang, Q. Liao, W. Li, Z. Tang, Q. Tong, X. Wang, F. Fang, M. R. De La Vega, Q. Ouyang, D. D. Zhang, S. Yu, and H. Zheng. "NRF2 Activation by Antioxidant Antidiabetic Agents Accelerates Tumor Metastasis." Science Translational Medicine 8.334 (2016). [http://www.ncbi.nlm.nih.gov/pubmed/?term=NRF2+activation+by+antioxidant+antidiabetic+agents+accelerates+tumor+metastasis] [PUBMED]
- 57Ramsey MR, Sharpless NE: ROS as a tumour suppressor? Nat Cell Biol. (2006) 8: 1213-1215 [PUBMED]
- 58Irminger-Finger I. Science of cancer and aging. J Clin Oncol. 2007 May 10;25(14):1844-51 [PUBMED]
- 59 a b Villarreal G, Zagorski J, Wahl Sm (January 29, 2003). Inflammation: Acute. In: ENCYCLOPEDIA OF LIFE SCIENCES. John Wiley & Sons, Ltd: Chichester [http://www.els.net/ [doi:10.1038/npg.els.0006101]]
- 60Fox JG, Wang TC. Inflammation, atrophy and gastric cancer. J Clin Invest. (2007) 117: 60-9 [PUBMED]
- 61Dobrovolskaia MA, Kozlov SV. Inflammation and cancer: when NF-ºB amalgamates the perilous partnership. Current Cancer Drug Targets. (2005) 5:325-44 [PUBMED]
- 62de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature Reviews. Cancer. (2006) 6: 24-37. [PUBMED]
- 63MacDonald N. Cancer cachexia and targeting chronic inflammation: a unified approach to cancer treatment and palliative/supportive care. J Support Oncol. (2007) 5(4): 157-62 [PUBMED]
- 64 a b Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med (2003) 348: 891-9. [PUBMED]
- 65 a b Campbell CL, Smyth S, Nontalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA. (2007) 297(18): 2018-24 [PUBMED]
- 66Chan AT. Aspirin, non-steroidal anti-inflammatory drugs, and colorectal neoplasia: future challenges in chemoprevention. Cancer Causes Control (2003) 14: 413-8 [PUBMED]
- 67Bertagnolli MM. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. Lancet Oncol. (2007) May;8(5):439-43. [PUBMED]
- 68Sandler RS, Halabi S, Baron JA, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med (2003) 348: 883-90. [PUBMED]
- 69Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med (2006) 355: 873-84 [PUBMED]
- 70Arber N, Eagle CJ, Spicak J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med (2006) 355: 885-95 [PUBMED]
- 71Leibowitz B, Qiu W, Buchanan ME, Zou F, Vernon P, Moyer MP, Yin XM, Schoen RE, Yu J, Zhang. BID mediates selective killing of APC-deficient cells in intestinal tumor suppression by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2014 Nov 18;111(46):16520-5. [PUBMED]
- 72Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. (2007) 356(21): 2131-42. [PUBMED]
- 73Couzin J. Drug Safety. FDA panel urges caution on many anti-inflammatory drugs. Science. (2005) 307(5713): 1183-5 [PUBMED]
- 74Markowitz SD. Aspirin and colon cancer--targeting prevention? N Engl J Med. (2007) 356(21): 2195-8. [PUBMED]
- 75Omer ZB, Ananthakrishnan AN, Nattinger KJ, Cole EB, Lin JJ, Kong CY, Hur C. Aspirin Protects Against Barrett's Esophagus in a Multivariate Logistic Regression Analysis. Clin Gastroenterol Hepatol. 2012 Jul;10(7):722-7. Epub 2012 Mar 15. [PUBMED]
- 76Baron JA. Aspirin and cancer: trials and observational studies. J Natl Cancer Inst. 2012 Aug 22;104(16):1199-200. [PUBMED]
- 77Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: a summary review to 2007. Recent Results Cancer Res. 2009;181:231-51. [PUBMED]
- 78Cooper K, Squires H, Carroll C, Papaioannou D, Booth A, Logan RF, Maguire C, Hind D, Tappenden P. Chemoprevention of colorectal cancer: systematic review and economic evaluation. Health Technol Assess. 2010 Jun;14(32):1-206. [PUBMED]
- 79Chan AT, Giovannucci EL, Meyerhardt JA, et al. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology 2008;134:21¿28.
- 80Chan AT, Giovannucci EL, Meyerhardt JA, et al. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA 2005;294:914¿923.
- 81Noreen F, Röösli M, Gaj P, Pietrzak J, Weis S, Urfer P, Regula J, Schär P, Truninger K.Modulation of age- and cancer-associated DNA methylation change in the healthy colon by aspirin and lifestyle. J Natl Cancer Inst. 2014 Jun 28;106(7). [PUBMED]
- 82Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women¿s Health Study: a randomized controlled trial. JAMA 2005;294:47¿55.
- 83Gann PH, Manson JE, Glynn RJ, et al. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst 1993;85:1220¿1224.
- 84Chan AT, Giovannucci EL. Primary prevention of colorectal cancer. Gastroenterology. 2010 Jun;138(6):2029-2043.e10. [PUBMED]
- 85Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E. Non-Steroidal Anti-Inflammatory Drugs and Prostate Cancer Risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol Biomarkers Prev. 2010 Oct 8. [PUBMED]
- 86Fowke JH, Motley SS, Smith JA Jr, Cookson MS, Concepcion R, Chang SS, Byerly S. Association of nonsteroidal anti-inflammatory drugs, prostate specific antigen and prostate volume. J Urol. 2009 May;181(5):2064-70 [PUBMED]
- 87 a b c Takkouche B, Regueira-Méndez C, Etminan M.Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Natl Cancer Inst. 2008 Oct 15;100(20):1439-47. [PUBMED]
- 88Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, Zhang WC, Li X. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009 Sep;117(1):141-50. [PUBMED]
- 89Bhattacharyya M, Girish GV, Ghosh R, Chakraborty S, Sinha AK. Acetyl salicyclic acid (aspirin) improves synthesis of maspin and lowers incidence of metastasis in breast cancer patients. Cancer Sci. 2010 Oct;101(10):2105-9. [PUBMED]
- 90Trabert B, Poole EM, White E, Visvanathan K, Adami HO, Anderson GL, Brasky TM, Brinton LA, Fortner RT, Gaudet M, Hartge P, Hoffman-Bolton J, Jones M, Lacey JV Jr, Larsson SC, Mackenzie GG, Schouten LJ, Sandler DP, O'Brien K, Patel AV, Peters U, Prizment A, Robien K, Setiawan WV, Swerdlow A, van den Brandt PA, Weiderpass E, Wilkens LR, Wolk A, Wentzensen N, Tworoger SS; Ovarian Cancer Cohort Consortium (OC). Analgesic Use and Ovarian Cancer Risk: An Analysis in the Ovarian Cancer Cohort Consortium. J Natl Cancer Inst. 2018 May 31. doi: 10.1093/jnci/djy100. PubMed [PUBMED]
- 91Merritt MA, Rice MS, Barnard ME, Hankinson SE, Matulonis UA, Poole EM, Tworoger SS. Pre-diagnosis and post-diagnosis use of common analgesics and ovarian cancer prognosis (NHS/NHSII): a cohort study. Lancet Oncol. 2018 Jul 17. pii: S1470-2045(18)30373-5. doi: 10.1016/S1470-2045(18)30373-5. PubMed [PUBMED]
- 92. (2020). Effect of aspirin on cancer incidence and mortality in older adults. Journal Of The National Cancer Institute. http://doi.org/10.1093/jnci/djaa114 (Original work published August 2020) [PUBMED]
- 93US Food and Drug Adminstration website. Accessed 6/20/2016. [http://www.fda.gov/]
- 94Routine Aspirin or Nonsteroidal Anti-inflammatory Drugs for the Primary Prevention of Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Annals of Internal Medicine. 2007 Mar;146(5):361-364. [PubMed]
- 95 Archived Final Recommendation Statement Aspirin/NSAIDs for Prevention of Colorectal Cancer: Preventive Medication Originally published on: December 30, 2013 Accessedd 1-7-2020 [USPS Archived Final Recommendation Statement on Aspirin/NSAIDS for the Prevention of Colorectal Cancer.]
- 96Jordan VC . Tamoxifen: a most unlikely pioneering medicine. Nat Rev Drug Discov. (2003) 2: 205-13 [PUBMED]
- 97 a b c Wozniak K, Kolacinska A, Blasinska-Morawiec M, Morawiec-Bajda A, Morawiec Z, Zadrozny M, Blasiak J. The DNA-damaging potential of tamoxifen in breast cancer and normal cells. Arch Toxicol. (2007) 81(7): 519-27 [PUBMED]
- 98 a b Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. (2007) 99(5): 350-6. [PUBMED]
- 99Hu H, Jiang C, Ip C, Rustum YM, Lu J. Methylseleninic acid potentiates apoptosis induced by chemotherapeutic drugs in androgen-independent prostate cancer cells. Clinical Cancer Research (2005). 11: 2379-2388. [PUBMED]
- 100Jordan, V. C., Allen, K. E. & Dix, C. J. Pharmacology of tamoxifen in laboratory animals. Cancer Treat. Rep. (1980) 64: 745-759. [PUBMED]
- 101Poirier MC, Schild LJ. The genotoxicity of tamoxifen: extent and consequences. Mutagenesis. (2003) 18: 395399 [PUBMED]
- 102Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomized prevention trial. Lancet. (2002) 360:81724. [PUBMED]
- 103 a b Richardson H, Johnston D, Pater J, Goss P. The National Cancer Institute of Canada Clinical Trials Group MAP.3 trial: an international breast cancer prevention trial. Curr Oncol. (2007) 14(3): 89-96 [PUBMED]
- 104Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl CancerInst (1998) 90: 1371-88 [PUBMED]
- 105Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomesthe NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. (2006) 295: 272741. [PUBMED]
- 106PETER H. RHEINSTEIN, M.D., J.D., M.S., U.S., Food and Drug Administration, Rockville, Maryland BARDIA AKBARI, PHARM.D., Robert Wood Johnson Medical School, New Brunswick, New Jersey Am Fam Physician. 1998 Jun 1;57(11):2865-2868. [Significant Drug FDA Approvals in 1997]
- 107Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP).Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007 Mar 23;56(RR-2):1-24. [PUBMED]
- 108U.S. Food and Drug Administration. News Release: FDA Approves New Indication for Gardasil to Prevent Genital Warts in Men and Boys. October 16, 2009. [http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm187003.htm]
- 109Centers for Disease Control and Prevention Newsroom. Accessed 11/1/2016. [http://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html]
- 110 a b Food and Drug and Administration Vaccines, Blood, & Biologics Accessed January 8, 2020 [FDA Website]
- 111Gardasil® Website Registered by Merck & Co., Inc. Site accessed January 8, 2020 [ Gardasil Website]
- 112Lowndes CM. "Vaccines for cervical cancer." Epidemiology and Infection. (2006) 134: 1-12. [PUBMED]
- 113Schmiedeskamp, MR and DR Kockler. "Human Papillomavirus vaccines." 2006, The Annals of Pharmacotherapy 40(7):1344-52 [PUBMED]
- 114Food and Drug and Administration Vaccines, Blood, & Biologics Accessed October 21st, 2009 [FDA Cervarix® Website]
- 115FDA website https://www.fda.gov/biologicsbloodvaccines/vaccines/approvedproducts/ucm426445.htm Accessed 10-7-2017
- 116US Food and Drug Administration. FDA Approves raloxifene to prevent osteoporosis. U.S. Department of Health and Human Services. Dec 10, 1997. [http://www.fda.gov/bbs/topics/ANSWERS/ANS00838.html]
- 117Harper DM, et al. "Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial." The Lancet (2004) Nov 13; 364:1757-65. [PUBMED]
- 118 Mulcahy N. GSK’s HPV Vaccine, Cervarix, No Longer Available in US. Medscape. October 24, 2016 [LINK]
- 119El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012 May;142(6):1264-1273.e1. [PUBMED]
- 120Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006 Oct;45(4):529-38. Epub 2006 Jun 23. [PUBMED]
- 121Vaccine. 2012 Mar 9;30(12):2212-9. Epub 2012 Jan 24. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012 Mar 9;30(12):2212-9. Epub 2012 Jan 24. [PUBMED]
- 122Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. Atkinson W, Wolfe S, Hamborsky J, eds. 12th ed. Washington DC: Public Health Foundation, 2011. http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hepb.pdf Accessed July 20, 2011. [http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/hepb.pdf]
- 123Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ; Advisory Committee on Immunization Practices (ACIP). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005 Dec 23;54(RR-16):1-31. [PUBMED]
- 124Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM Jr, Janssen RS, Ward JW; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006 Dec 8;55(RR-16):1-33; quiz CE1-4. [PUBMED]
