Vincristine sulfate liposome injection
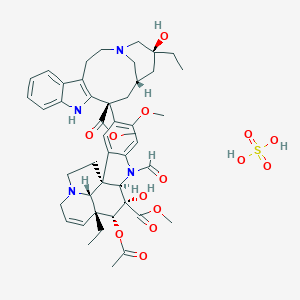
Marqibo® (Vincristine sulfate liposome injection) was approved by the FDA in 2012. It is used to treat adults with Philadelphia chromosome negative (Ph-) acute lymphoblastic leukemia (ALL), a rare type of leukemia. This drug is used in patients whose leukemia has relapsed/returned 2 or more times or whose disease has progressed after 2 or more anti-leukemia therapies.1
- 1 Marqibo Prescription Information. 2016. http://www.marqibo.com
Vincristine sulfate liposome injection (Marqibo®) is a sphingomyelin/cholesterol liposome-encapsulated form of a vinca alkaloid. The liposome capsule is made from materials similar to that of cell membranes and serves as a drug delivery vehicle for vincristine sulfate. To be active, the vinca alkaloid must be released from the liposome. Non-liposomal vincristine sulfate binds tubulin and alters tubulin polymerization, disrupting normal microtubule structure and function. Non-liposomal vincristine sulfate stabilizes the spindle apparatus and prevents chromosome segregation, inhibiting metaphase and mitosis.1
- 1 Marqibo Prescription Information. 2016. http://www.marqibo.com
