Toremifene
Toremifene is used in treating metastatic breast cancers in postmenopausal women whose cancers have either not been classified as either estrogen receptor positive or negative, or in those that have been determined to be estrogen receptor positive. Toremifene is taken in tablet form usually just once a day.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Toremifene citrate (Fareston®) is similar to Tamoxifen, in that molecules bind to estrogen receptors in breast cancers. By blocking estrogen molecules from binding sites, toremifene prevents estrogen's growth stimulating effects in the tumor. Drugs that act in this way are referred to anti-estrogens because they block the effects of estrogen in certain tissues.1
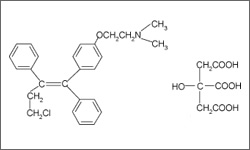
The diagram below shows the 3D molecular structure of Toremifene.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include hot flashes and sweating, nausea and vomiting, vaginal discharge, dizziness, swelling. Less commonly, some patients may experience vaginal bleeding and in the event of this should contact their physician. There also is concern that in some tissues and organs of the body, other than the breast, where toremifene may act as an estrogen rather than an anti-estrogen, cell proliferation may be induced that could result in tumor formation. It is still unclear if this drug may cause the proliferation of endometrial (inner uterus) cells.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
