Ixabepilone
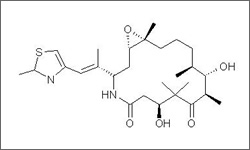
Ixempra® (ixabepilone) was approved by the FDA in 2007. Ixabepilone is used in combination with capecitabine to treat metastatic or locally advanced breast cancer after treatments with an anthracycline and a taxane have failed. Ixabepilone is used by itself (as a monotherapy) to treat metastatic or locally advanced breast cancer after treatments with an anthracycline, a taxane, and a capecitabine have failed. Examples of anthracyclines include doxorubicin and epirubicin. Examples of taxanes include paclitaxel and docetaxel.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Ixabepilone (Ixempra®) is a microtubule function inhibitor and a member of the epothilone class of anticancer agents. Ixabepilone binds to beta-tubulin subunits on microtubules, prohibiting them from depolymerizing during mitosis. This halts cell division and eventually leads to cell death.1
The above diagram shows the 3D conformer Ixabepilone.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Fatigue, myalgia (muscle pain) or arthralgia (joint pain), alopecia (hair loss), nausea, vomiting, stomatitis (inflammation of the mucous linings of the mouth) mucositis (inflammation of the mucous linings of the digestive tract), and diarrhea.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
The most common toxicities associated with ixabepilone are peripheral neuropathy, myelosuppression (particularly of white blood cells) and hypersensitivity reactions. Due to the potential for alteration of ixabepilone metabolism, patients taking strong CYP3A4 inhibitors or inducers should be treated with caution. Ixabepilone has potential to harm a developing fetus, therefore prospective mothers should be warned of this risk and women should avoid becoming pregnant while receiving treatment ixabepilone treatment.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
