Cetuximab
Cetuximab is approved for the treatment of: advanced squamous cell carcinoma of the head and neck (in combination with radiation). Recurrent or metastatic squamous cell carcinoma of the head and neck (after failure of platinum-based therapy). Metastatic, EGFR-expressing colorectal cancer (after failure of irinotecan and oxaliplatin). Metastatic, EGFR-expressing colorectal cancer (in combination with irinotecan)1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Cetuximab (Erbitux®) binds to the human epidermal growth factor receptor (EGFR) to inhibit the binding of epidermal growth factor (EGF). This action prohibits the activation of receptor-associated kinases within the cell, resulting in an inhibition of cell growth, promotion of apoptosis and decrease in the production of matrix metalloproteases (MMPs) and vascular endothelial growth factor (VEGF)--two factors necessary for tumor maintenance and growth.1
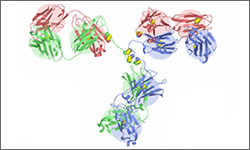
The 3D diagram below shows the molecular structure of Cetuximab complexed with FH3 meditope variant.
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Side Effects include: infusion reactions (airway obstruction, low blood pressure, loss of consciousness or cardiac arrest), skin rash, inflammation or abscess formation, headache, diarrhea, infection. The most frequent side effects are acne-like rashes, weakness or discomfort, diarrhea, and nausea.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
The most serious toxicities reported with cetuximab are cardiopulmonary arrest, pulmonary toxicity and electrolyte abnormalities (low potassium, magnesium and/or calcium levels). Due to potential harm to a developing fetus, cetuximab should be used with caution in pregnant women and those on cetuximab treatment should avoid becoming pregnant and should not breastfeed.
