Capecitabine
Capecitabine is a suppressor of bone marrow activity and is frequently used in treatment of breast and bowel cancers. It is important to monitor blood cell and platelet counts throughout the duration of treatment with blood tests before each infusion. Capecitabine may also have toxic effects the hepatic (liver) system which, require liver function to be monitored routinely. Also, cardiac function must be monitored to avoid irreversible effects of toxicity. Evaluations of the function of these systems should be made before each infusion. Capecitabine should not be taken by women who are pregnant and patients should not become pregnant while using this drug, as it may have harmful affects on the developing fetus. This drug may have effects on fertility after treatment has ended. 1
- 1 Xeloda.. Prescribing Information. Roche Pharmaceuticals. March, 2015. [https://www.gene.com/download/pdf/xeloda_prescribing.pdf]
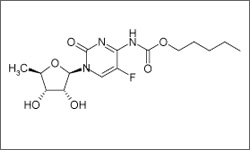
Capecitabine (Xeloda®) is an antimetabolite that is changed to 5-fluorouracil inside the body. It inhibits cell division and interferes with RNA and protein processing.
The diagram below shows the 3D molecular structure of Capecitabine.
Common side effects include: nausea and vomiting, mouth sores, abdominal pain, diarrhea, loss of appetite, and changes in skin. Less common side effects include temporary bone marrow suppression, hair loss, increased tear production, headache and dizziness. Side effects may be different or more severe if capecitabine is taken with other drugs. Capecitabine is a suppressor of bone marrow activity.
