Alemtuzumab
Alemtuzumab is used in the treatment B-cell chronic lymphocytic leukemia (B-CLL) that have already been treated with certain chemotherapy drugs. Alemtuzumab is administered as an IV infusion.
Alemtuzumab was withdrawn from the U.S. and European markets in 2012.1 The drug was then approved as Lemtrada® for the treatment of relapsed multiple sclerosis.2
- 1 Sanofi withdraws Campath in U.S. and EU. Published August 21, 2012 Accessed January 8, 2020 [PharmaTimes Online]
- 2 FDA Approval of Lemtrada on Drugs.com Published November 14, 2014. Accessed January 8, 2019 [Drugs.com Lamtrada Page]
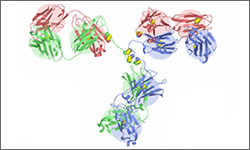
Alemtuzumab (Campath®) is a monoclonal antibody directed against the CD52 protein. The CD52 protein is present normal and cancerous B and T cells and other immune cells. It is thought that after the antibody binds to the surface of the cells, it stimulates the destruction of the tagged cells, through antibody-dependent lysis. Note that the image shown is a general structure of an antibody. There are four proteins bound together in a 'Y' shape. The two top tips of the Y are where the antibody binds with its target. This means that each antibody molecule can bind to two identical target regions.
The structure below shows the antibody to Campath-1H Humanized Fab.
Alemtuzumab can affect normal cells of the blood, which can result in anemia, increased risk of bleeding, and infection. For this reason blood counts will be monitored throughout the duration of treatment. Birth control should be used during treatment and at least six months after treatment has ended for both men and women. Infusion reactions may also occur. These reactions are much more likely to occur during the first week of treatment. Alemtuzumab may result in a weakened immune system. 1. Symptoms may include fever, chills, nausea, vomiting, and low blood pressure. Other side effects may include: rash, fatigue, shortness of breath, coughing, diarrhea, headache, loss of appetite, itching, sweating, dizziness, and abdominal pain.2
- 1 Alemtuzumab (marketed as Campath) Information. FDA. [http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm082681.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=Alemtuzumab&utm_content=1]
- 2 Prescribing Information. Compath [http://www.campath.com/]
