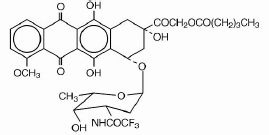
Valrubicin
Valstar is recommended at a dose of 800 mg administered intravesically once a week for six weeks. Administration should be delayed at least two weeks after transurethral resection and/or fulguration. For each instillation, four 5 mL vials (200 mg valrubicin/5 mL vial) should be allowed to warm slowly to room temperature, but should not be heated. Twenty milliliters of Valstar should then be withdrawn from the four vials and diluted with 55 mL 0.9% Sodium Chloride Injection, USP providing 75 mL of a diluted Valstar solution. A urethral catheter should then be inserted into the patient''s bladder under aseptic conditions, the bladder drained, and the diluted 75 mL Valstar solution instilled slowly via gravity flow over a period of several minutes. The catheter should then be withdrawn. The patient should retain the drug for two hours before voiding. At the end of two hours, all patients should void. (Some patients will be unable to retain the drug for the full two hours.) Patients should be instructed to maintain adequate hydration following treatment. Patients receiving Valstar for refractory carcinoma in situ must be monitored closely for disease recurrence or progression. Recommended evaluations include cystoscopy, biopsy, and urine cytology every 3 months.
Valstar® (valrubicin) Sterile Solution for Intravesical Instillation is intended for intravesical administration in the urinary bladder. It is supplied as a nonaqueous solution that should be diluted before intravesical administration. Each vial of Valstar contains valrubicin at a concentration of 40 mg/mL in 50% polyoxyl castor oil/50% dehydrated alcohol, USP without preservatives or other additives. The solution is sterile and nonpyrogenic.
Valrubicin is an anthracycline that affects a variety of interrelated biological functions, most of which involve nucleic acid metabolism. It readily penetrates into cells, where it inhibits the incorporation of nucleosides into nucleic acids, causes extensive chromosomal damage, and arrests cell cycle in G2. Although valrubicin does not bind strongly to DNA, a principal mechanism of its action, mediated by valrubicin metabolites, is interference with the normal DNA breaking-resealing action of DNA topoisomerase II.
Side effects of Valstar include: bladder irritation, with symptoms such as pain, spasm, and frequent urge to urinate, usually occurs. This medication usually will cause your urine to turn a reddish color. This is a normal, harmless effect of the drug and should not be mistaken for blood in your urine. If any of these effects persist or worsen after 24 hours, contact your doctor right away. Infrequent side effects include nausea, abdominal/stomach pain, diarrhea, headache, weakness, dizziness, or back pain. If any of these effects persist or worsen, tell your doctor.
