Pamidronate
Hypercalcemia of malignancy (HCM) is a condition in which calcium is released into the blood stream from the bones in excess. HCM can be caused by factors that tumors release into the blood that in turn circulate throughout the body and activate cells that degrade bone, or also can result from metastases in bone from solid and myelogenous cancers that locally cause bone loss and calcium release by stimulating bone degrading cells. Pamidronate disodium (Aredia®) is used to treat hypercalcemia of malignancy. Aredia is administered as an IV infusion and its administration is accompanied by other cancer fighting drugs.1
- 1 Aredia.. Prescribing Information. Novartis Pharmaceuticals Corporation. June, 2012. https://www.old.health.gov.il/units/pharmacy/trufot/alonim/aredia_dr_1347250141170.pdf
Although the mechanism by which Aredia acts is not completely understood, it is thought that there are a couple of mechanisms may be responsible for its action. Aredia adheres to a particular form of calcium crystal in the bone and directly blocks it from being broken down and released into the blood stream. It is also possible that it inhibits the particular cells that are responsible for bone degradation, osteoclasts.1
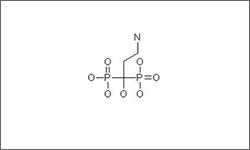
The structure below shows Pamidronate.
- 1 Aredia.. Prescribing Information. Novartis Pharmaceuticals Corporation. June, 2012. https://www.old.health.gov.il/units/pharmacy/trufot/alonim/aredia_dr_1347250141170.pdf
Common side effects include fluid retention, generalized body pain, constipation, nausea and vomiting. It is important that patients undergo regular tests to evaluate the levels of certain important factors in the blood that may become depleted with treatment. Because Aredia can affect the kidneys tests should be done to monitor kidney function. 1
- 1 Aredia.. Prescribing Information. Novartis Pharmaceuticals Corporation. June, 2012. https://www.old.health.gov.il/units/pharmacy/trufot/alonim/aredia_dr_1347250141170.pdf
