Thalidomide
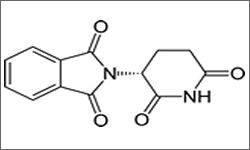
THALOMID®, in combination with dexamethasone, is indicated for the treatment of patients with newly diagnosed multiple myeloma. Thalidomide is also FDA-approved for treatment of ENL and MDS.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
The mechanisms that characterize this antiangiogenic agent are not fully understood, however it is known that Thalidomide inhibits TNF alpha synthesis. In addition to this, Thalidomide may also exert antiangiogenic effects, which inhibit BFGF and VEGF.1
The diagram below shows the 3D molecular structure of Thalidomide.
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
The side effects of thalidomide include blood clots (thromboembolic events), drowsiness, damage to the nervous system (peripheral neuropathy), reduced white blood cell counts (neutropenia), constipation, and drops in blood pressure upon standing (orthostatic hypotension).1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Thalidomide causes severe birth defects (i.e. it is a potent teratogen). Women who are pregnant or who might become pregnant can not use thalidomide unless they follow very strict guidelines to prevent pregnancy.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
