Docetaxel
Malignancies for which docetaxel is most frequently used include: breast cancer (advanced or metastatic) and non-small cell lung cancer. Docetaxel is also used in the treatment of prostate, gastric, and head and neck cancers. Docetaxel is administered as an intravenous infusion.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Docetaxel (Taxotere®) is a semi-synthetic drug and is closely related to paclitaxel (Taxol®, Onxol®). The original chemical was isolated from extracts taken from yew trees. Through the process of binding, Docetaxel inhibits the breakdown of microtubules, which interfere with cell division and cause cell death. Docetaxel is a cell cycle specific agent that has activity in the mitotic phase.1
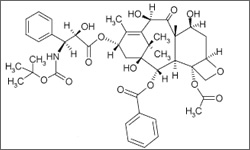
The diagram below shows the 3D molecular structure of Docetaxel.
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include: bone marrow suppression, fluid retention, hair loss, infection due to bone marrow suppression, diarrhea and rash. Side effects may be different or more severe if docetaxel is being administered with other cancer treatments. Treatment with docetaxel is usually accompanied with steroids in order to prevent severe fluid retention. However, fluid retention still occurs in some cases. Docetaxel is a strong suppressor of bone marrow activity. It is important to monitor blood cell and platelet counts throughout the duration of treatment.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Docetaxel should not be taken by women who are pregnant and patients should not become pregnant while using this drug, as it may have harmful affects on the developing fetus. Patients with abnormal lover function should also be cautious, as they are at a higher risk of toxicity.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
