Decitabine
Decitabine is approved to treat myelodysplastic syndromes (MDS) of many different subtypes and is only administered intravenously. Patients are often premedicated with anti-nausea medications in order to limit poor reactions post-treatment. Decitabine may cause severe reactions affecting the lungs, heart, brain, liver and bilirubin.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Decitabine (Dacogen®) is a nucleoside analog that incorporates directly into DNA and is believed to inhibit DNA methyltransferase. This may function in cancer cells to restore normal function to genes necessary for the control of cell proliferation and promote apoptosis of damaged cells.1
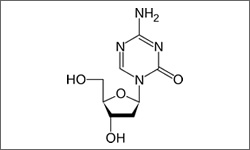
The molecular structure above shows the 3D conformer Decitabine.
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Decitabine has the potential to harm a developing fetus, therefore women should avoid becoming pregnant during treatment and pregnant women should be warned of this risk. Men should not father a child during or immediately following treatment. Additionally, new mothers should discontinue nursing infants during treatment.1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
