Leuprolide acetate implant
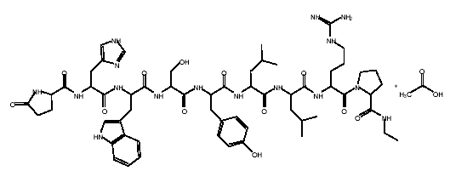
An LH-RH (GnRH) analog, leuprolide may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids), to treat precocious puberty, and to control ovarian stimulation in In Vitro Fertilization (IVF). It is considered a possible treatment for paraphilias. Leuprolide has been tested as a treatment for reducing sexual urges in pedophiles and other cases of paraphilia. As of 2006 Leuprolide was under investigation for possible use in the treatment of mild to moderate Alzheimer's disease. It also used for treatment of steroid abuse. Leuprolide, along with triptorelin and goserelin, are often used to delay puberty in transgender youth until they are old enough to begin hormone replacement therapy. They are also sometimes used as superior alternatives to anti-androgens like spironolactone and cyproterone acetate for suppressing testosterone production in trans women.
The recommended dose of Viadur® is one implant for 12 months. Each implant contains 65 mg leuprolide. The implant is inserted subcutaneously in the inner aspect of the upper arm and provides continuous release of leuprolide for 12 months of hormonal therapy. Viadur® must be removed after 12 months of therapy. At the time an implant is removed, another implant may be inserted to continue therapy.
Leuprolide acetate, an LH-RH agonist, acts as a potent inhibitor of gonadotropin secretion when given continuously and in therapeutic doses. Animal and human studies indicate that after an initial stimulation, chronic administration of leuprolide acetate results in suppression of ovarian and testicular steroidogenesis.
In humans, administration of leuprolide acetate results in an initial increase in circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to a transient increase in concentrations of gonadal steroids (testosterone and dihydrotestosterone in males, and estrone and estradiol in premenopausal females). However, continuous administration of leuprolide acetate results in decreased levels of LH and FSH. In males, testosterone is reduced to castrate levels. These decreases occur within 2 to 4 weeks after initiation of treatment.
Less common side effects of Viadur for adults include: fast or irregular heartbeat. Rare side effects of Viadur include: bone, muscle, or joint pain; fainting; fast or irregular breathing; numbness or tingling of the hands and feet; puffiness or swelling of the eyelids or around the eyes; skin, rash, hives, or itching; sudden, severe decrease in blood pressure and collapse; tightness in the chest; and troubled breathing.
More common side effects of Viadur for male adults only include: arm, back, or jaw pain; bloody or cloudy urine; blurred vision; burning while urinating; chest pain or discomfort; chest tightness or heaviness; difficult or labored breathing; difficult, burning, or painful urination; difficulty with moving; dizziness; frequent urge to urinate; headache; increased urge to urinate during the night; muscle pain or stiffness; nausea; nervousness; pain in the joints; pale skin; pounding in the ears; slow or fast heartbeat; sweating; troubled breathing with exertion; unusual bleeding or bruising; unusual tiredness or weakness; and waking to urinate at night.
