Vinblastine
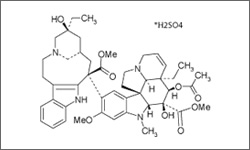
Vinblastine is used in the treatment of Hodgkin's and non-Hodgkins lymphoma, testicular cancer, breast cancer, Kaposi's sarcoma and renal cell carcinoma. The treatment is administered intravenously.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Vinblastine (Velban®, Velsar®) prevents the cancer cells from undergoing mitosis. It does this by inhibiting the formation of spindle fibers. Spindle fibers are responsible the alignment of chromosomes and the separation of the chromosomes in anaphase. Vinblastine blocks the tubulin monomers from forming microtubules. Without proper microtubule formation cell division is not possible. Like all of the vinca alkaloids, this drug also affects cell division in normal cells, explaining many of the side effects seen. 1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include bone or muscle pain, nausea and vomiting, alopecia (loss of hair), sores in mouth and on lips, swelling of feet or lower legs. Less common side effects include: dizziness, double vision, drooping eyelids, headache, jaw pain, mental depression, numbness or tingling in fingers and toes, pain finger and toe pain, testicle pain, waking difficulties, weakness, blood in urine or stools, and unusual bleeding or bruising.1
- 1 Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
