Lomustine
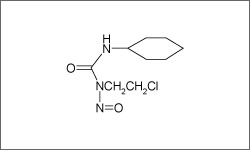
Lomustine forms reactive metabolites which cause cross-linking of DNA and inhibition of DNA synthesis through a process that interferes with the creation of DNA, RNA and proteins.1
The molecular structure above shows the 3D conformer Lomustine.
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
Common side effects include myelosupression, nausea and vomiting, birth defects, loss of appetite, weight loss, weakness, excessive bleeding, difficulty walking, diarrhea, lethargy. Women who are pregnant or thinking of becoming pregnant should not take this drug as birth defects are a possibility. There is also an increased tendency to bleed making it necessary that the person not take aspirin as it 'thins' the blood, further increasing bleeding potential. Lomustine may have a negative effects on the the immune system. Because of this, patients should not receive immunizations while being treated. 1
- 1Chu, E., & DeVita, V. T. (2015). Physicians' cancer chemotherapy drug manual 2015. Burlington, MA: Jones & Bartlett Learning.
